NEWS
Hot off the press
The latest industry news
ASA upholds complaint against Northwest cosmetic training company
The Advertising Standards Authority (ASA) has ruled in favour of a complaint lodged against a cosmetic training company based in the Northwest of England. The complaint, brought forth by the Joint Council for Cosmetic Practitioners (JCCP), highlighted misleading advertising practices. The contested advertisement, posted on Facebook by the training company, promoted a course titled ‘Pathway to Aesthetics’ as ‘fully accredited’ for £299. The ASA determined that the advertisement was likely to mislead prospective students and violated the Committees of Advertising Practice (CAP) Code.
The JCCP argued that the promotional post was deceptive, as it failed to adequately communicate the nature, requirements, and qualifications associated with the aesthetics procedures training course. The claim of being ‘fully accredited’ and ensuring students were ‘fully qualified’ was also found to be misleading by the ASA. In its ruling, the ASA cited the NHS’s recommendation to avoid practitioners with only short training courses due to potential complications and risks. It also emphasised the importance of checking professionals’ accreditation on a voluntary register endorsed by the Professional Standards Authority (PSA), including the JCCP.
The ASA acknowledged the distinction between Continuing Professional Development (CPD) training and recognised qualifications, noting that the advertised course only provided a CPD certificate, not a recognised qualification. The finding stated that the advertisement’s promise of students becoming ‘fully qualified’ was misleading, as it was an introductory course, requiring additional training and experience for independent practice.
As a result of the upheld complaint, the aesthetics training company has been cautioned against implying that its courses confer recognised qualifications.
MHRA issues warning regarding unsafe counterfeit weight loss pens
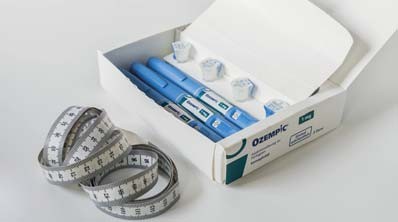
The Medicines and Healthcare products Regulatory Agency (MHRA) is cautioning the public against the purchase of pre-filled pens falsely claiming to contain Ozempic (semaglutide) or Saxenda (liraglutide). These potentially harmful counterfeit pens have surfaced in the UK, and the MHRA emphasises the importance of obtaining these medicines solely through a valid medical prescription. This comes after BBC Three’s documentary “The Skinny Jab Uncovered”, in which BBC News shared details of an undercover investigation into beauty practitioners illegally selling semaglutide or knock-off alternatives, nicknamed ‘skinny injections’ to customers, despite not being medical professionals. The British Association of Beauty Therapy and Cosmetology (BABTAC) has also issued a warning of the dangers of illegally obtaining the drug. Since January 2023, the MHRA has seized 369 potentially fake Ozempic pens and received reports of counterfeit Saxenda pens in the possession of the public in the UK, obtained through non-legitimate channels. The MHRA has received reports of a small number of individuals being hospitalised after using potentially fake pens. Serious side effects, including hypoglycemic shock and coma, suggest that these pens may contain insulin rather than the intended semaglutide.

Under-18s visiting Wales for botulinum toxin treatments
It has been reported by the BBC that under-18s are travelling to Wales for botulinum toxin injections. Since October 1, 2021, it has been a criminal offence to administer botulinum toxin to a person under 18 in England, after the Botulinum Toxin and Cosmetic Fillers (Children) Act came into place. This act, however, did not cover Scotland, Ireland, or Wales.
Save Face shared with the BBC that they have received several reports of minors travelling across the border for treatment and that what they’re seeing is “literally the tip of the iceberg.” Ashton Collins, director of Save Face, thought it would be a “no brainer” for Wales to follow England’s footsteps after she worked with MP Laura Trott to bring in the act.
The Welsh government has responded and said botulinum toxin is a prescription-only medicine and that it was the responsibility of the qualified prescriber to ensure the product is “given safely and in accordance with accepted professional standards and in the patient’s best interests.”
BABTAC revises Best Practice Guide for practitioners treating patients with cancer
To coincide with Breast Cancer Awareness Month, the British Association of Beauty and Cosmetology (BABTAC) has launched its Best Practice Guide. Compiled to ensure that therapists feel confident in offering the best service, the guide includes information on clients’ rights, insurance considerations, training recommendations and clarification around non-invasive and invasive treatments.
When it comes to treatments, although there are various options beneficial in supporting the well-being of cancer patients, the disease can present certain contraindications, so whether it be through physical or reactionary changes to the body because of medical treatment, adaptation by therapists may be required.
“As a professional association, BABTAC continuously seeks ways to support our members and further the industry,” said BABTAC chair and chief executive Lesley Blair. “Taking into account all of the information provided and researched, we have developed our stance for treating clients who have or are recovering from cancer which provides the best practice tools needed to support a client emotionally and physically through what can be a traumatic experience.
“Our guidelines are reviewed yearly and updated in light of any new information or research and are also endorsed by both Cancer Research and Macmillan.”
The BABTAC Best Practice Guide on treating clients with cancer is available now to all BABTAC members.
Comparing rapid acoustic pulse rates in cellulite treatment
A study has been published exploring the efficacy and safety of a novel approach to cellulite treatment, known as acoustic subcision, by comparing two different rapid acoustic pulse rates. The research aims to determine whether a higher treatment rate could lead to more significant improvements in the appearance of cellulite.
Titled “Comparing safety and efficacy of acoustic subcision at two different rapid acoustic pulse rates to improve the appearance of cellulite,” the study was led by Dr Brian S Biesman, clinical assistant professor at Vanderbilt University Medical Center, who is a renowned expert in minimally invasive cosmetic and reconstructive eyelid and facial surgery. Findings were published in the official journal of the American Society for Laser Medicine and Surgery. (ASLMS).
A total of 15 female participants received rapid acoustic pulse treatments on both their left and right buttocks and thighs. The treatment involved the use of either a 100 Hz or 50 Hz pulse rate, with the same number of pulses administered on each side. The outcomes of both the 100 Hz and 50 Hz treatments were evaluated through blind independent physician photo reviews, participant satisfaction ratings, and assessments of ‘cellulite dimple - at rest’ scores.
“Delivering rapid acoustic pulses at 100 Hz demonstrated safety and efficacy similar to that of 50 Hz,” notes Dr Biesman. “This led to substantial improvements in the appearance of cellulite with minimal discomfort, and high satisfaction was reported among participants. This study is significant as it showcases the potential for more efficient acoustic subcision treatments, maintaining both safety and efficacy.”
JCCP announces new approved education provider
The Joint Council for Cosmetic Practitioners (JCCP) has announced that formal approval has now been awarded to the University of Manchester’s Post Graduate Diploma/MSc in Skin Ageing and Aesthetic Medicine regarding dermal fillers and injectable toxins.
The diploma has now been added to the JCCP’s Register of Approved Qualif ications. The University of Manchester is now entitled to display the JCCP charter marks in accordance with the JCCP protocol for the use of the same.
The JCCP is at the forefront of the call for regulation within the aesthetic sector and has built on the previous Health Education England (HEE) approved framework by setting education and training standards and competency frameworks for the industry in collaboration with the Cosmetic Practice Standards Authority (CPSA).
The standards and competencies set down by the JCCP and the CPSA may be accessed via the JCCP website and are presented as the JCCP Competence Framework (2018). These standards are designed for use by higher education institutions (universities) and by Ofqual and SQA recognised awarding bodies to assist in the development of qualifications and education/training programmes in order to demonstrate alignment with the Council’s published standards.
Cutera announces new Centre of Excellence in Ireland
Mr Kambiz Golchin, an esteemed ENT Surgeon and visionary in the field, is to spearhead a new Cutera Centre of Excellence at Beacon Dermatology Clinic. This collaboration marks an exciting milestone for the medical aesthetics device manufacturer, as it expands its presence and offers advanced aesthetic solutions to customers throughout Ireland.
The brand confirms that the new Centre of Excellence will offer state-of-the-art equipment and advanced training opportunities for physicians and practitioners in the field. It will serve as a hub for innovation and knowledge sharing, promoting advancements in non-invasive aesthetic treatments.
“This new Centre of Excellence represents an exciting development for both Cutera and our valued customers in Ireland, providing an exceptional learning environment for physicians, practitioners, and individuals seeking in-depth insights into the latest advancements in medical aesthetics,” said Sam Keene, regional manager for the UK and Ireland. “We are thrilled to collaborate with esteemed experts like Mr Kambiz Golchin in our shared commitment to fostering excellence within the medical aesthetics community.”
DermaFocus partners with training company to offer polynucleotide training
In a move to provide increased training opportunities on polynucleotides, DermaFocus has partnered with training provider Cosmetic Courses.
From January 2024, doctors, dentists, nurses, and prescribing pharmacists will be able to attend standard training to learn about the innovative Plinest and Newest polynucleotides from renowned regenerative aesthetic manufacturer Mastelli. Training will be held at Cosmetic Courses’ training sites in London, Buckinghamshire, Nottingham and Leeds.
Following successful completion of training, delegates will receive CPD certification and unlimited support from the DermaFocus team to help integrate polynucleotides into clinics.
“It is an honour to work with Cosmetic Courses to expand our polynucleotides training,” said the executive director of DermaFocus, Milad Bemana.
“This partnership will give aesthetic practitioners increased opportunities to start their regenerative aesthetics journey and integrate these highly effective products into their clinic offering in 2024.”
Teoxane receives new medical device regulation certifications
Teoxane has been granted Medical Device Regulation (MDR) certification, making it the first company to have its entire product portfolio of sterile, injectable, hyaluronic acid-based products recognised for their safety and quality in treating wrinkles.
The CE certification, as part of the MDR implementation, is designed to ensure the safety, efficacy and quality of medical devices marketed in the European Union. Its objectives include improving traceability throughout the supply chain and enhancing the transparency of data relating to these devices. Teoxane demonstrated the quality and safety of Teosyal Puresense and Teosyal RHA products through 14 clinical studies conducted in Europe and the United States.
Dr Aamer Khan appointed by ABG Lab as global KOL
Aesthetic doctor, regenerative medicine expert, and Harley Street Skin Clinic clinical director and co-founder Dr Aamer Khan (MB ChB) has been appointed as ABG Lab’s global key opinion leader (KOL).
Dr Kahn joins ABG Lab’s growing team of global KOLs alongside Dr Michael Kane, and Professor Yana Yutskovskaya at a period of global growth for the brand.
“We’re delighted to announce our partnership with Dr Khan and are thrilled that he will be offering ABG Lab’s cutting-edge skincare technologies and intradermal injections at his Harley Street Skin Clinic,” says president and chief executive of ABG Lab, Dr Elina Tester. “Our vision to bring the very best in regenerative medicine to patients across the globe aligns perfectly with that of Dr Khan, and this partnership signifies our ongoing commitment to collaborating with the very best in the industry and those who share our values and passion for offering patients the epitome of technology and technique.”
Success in Aesthetics Business announces partnership with Aesthetics Business Conference
Success in Aesthetic Business (SIAB) and Aesthetics Business Conference (ABC) have announced an aesthetic business support partnership in 2024.
The collaboration promises to offer comprehensive access to industry specialists in business skills across all regions. This initiative will unfold over a 12-month period, culminating in the ABC event at the end of 2024. The event is set to feature masterclasses in business skills and legislation, further enriching the learning and career development opportunities for industry professionals.
Besides the live talks and the one-to-one sessions, delegates will also get access to educational talks post-event, via online webinars. SIAB, in partnership with DSL Consulting Ltd and DH Training, has run a series of successful business support days for aesthetic practitioners to enhance their business skills and enable practitioners to take their business to the next level. Hamilton Fraser, owner of ABC, has been providing the sector with leading insurance products for over 25 years, providing business education and support to the industry, making SIAB and ABC a great pairing to offer support to aesthetic business owners.
Harpar Grace International debuts the ‘Skin Squad’
Harpar Grace International debuted the ‘Skin Squad’ this month, a new mobile team bringing bespoke activations, events, and educational experiences directly to its UK and Irish accounts.
The client-centric approach is aimed at nurturing growth and offering comprehensive support to the aesthetic clinics and wellbeing destinations stocking HGI’s portfolio of skincare, haircare, and well-being solutions.
Clinic and studio managers looking to scale up their in-house resources can call upon the HGI ‘Skin Squad’ for assistance. Supporting iS Clinical, Déesse Pro, RevitaLash Cosmetics, and Totally Derma, the team is ready to provide refresher training on both new and signature products and treatments, provide VIP and influencer treatments, manage social activations (including live content generation) and support customer events for both prospects and loyal patrons.
News in Brief
BTL Aesthetics announces new managing director
Aesthetic and medical device brand BTL Aesthetics has announced the appointment of Tim Taylor as managing director. Under his leadership, the business aims to build upon BTL’s market momentum with the continued effort to introduce revolutionary aesthetic, physiotherapy, and well-being energy devices.
ABG Lab announces new distributor
ABG Lab has announced that it has added UK wholesaler and distributor Rosmetics Aesthetics to its global distributor portfolio. The company will distribute ABG Lab’s range of regenerative therapy intradermal injectables to medical aesthetics businesses across the UK.
Dr Sophie Shotter announces guest residency in Dubai clinic
Dr Sophie Shotter has announced a new regular guest residency at Biolite Aesthetic Clinic, bringing her finesse, knowledge, expertise, and award-winning full-face rejuvenation techniques to the heart of the Middle East. With regular guest clinics to be revealed for next year, clients can look forward to dates becoming available in January, April, and June of 2024.
ABC Medical announces new distribution partnerships
ABC Medical has announced the addition of three new partnerships with Cynosure, Deleo, and EMA Aesthetics Ltd. The new distribution agreements are part of ABC Medical’s ongoing diversification in the field of medical aesthetic devices, providing each brand with support, resources, and training from some of the industry’s leading names to boost growth and market share.
Menopause in Aesthetics shares 2024 conference return date
Menopause in Aesthetics (MiA) is set to return in 2024 with more expert speakers, expanded content, and a larger venue. Taking place on Friday, February 9, 2024, at the renowned Business Design Centre, London, MiA is a conference for anyone looking to provide menopause support in their aesthetic clinics.
Liz McKeon announces new retail course for clinic owners
Business expert Liz McKeon has launched a new programme providing insights to business owners on how best to leverage the opportunities that retail sales present to attract and retain exceptional star team members. The programme aims to help clinic owners identify how they can structure their retail department and attract, retain, and increase loyalty to their business while, in turn, increasing client retention and profit per visit.
BABTAC and CIBTAC appoint new board member
The British Association, and International Confederation, of Beauty Therapy and Cosmetology have announced the appointment of Keith Conniford to the boards. Conniford is passionate about achieving regulation. He brings with him a wealth of skills and experience including education, training, HR, and retail further strengthening the existing board team as well as helping continue to promote the very best standards within the industry.
