CLINICAL
Is it time to rethink lab skin testing?
Deputy editor Kezia Parkins speaks to biotech startup Keratify, which is aiming to resolve the limitations of current laboratory skin testing with an ex vivo solution to create safer and more effective skincare.
According to dermato-endocrinologist Dr Rosalind Hannen, current in vitro and ex vivo models, designed A to replace animal models in dermatological pre-clinical testing, are deeply flawed for their lack of ability to fully replicate the human response. “When it comes to ex vivo, I have found that maintaining human skin in the lab is really suboptimal because the skin barrier would break down,” the multi-award-winning chief executive of Keratify explains.
“This is particularly true for healthy skin tissue — it starts to look like it’s wounded and behaves more like diseased skin.”
Similarly, the current 3D in vitro skin models also come with their limitations. “What this means is that current preclinical testing can have high error rates,” Hannen adds.
The main difference between in vitro and ex vivo tests is that the former is simply a cell system established in a cell culture laboratory (in vitro is Latin for ‘within glass’) whereas the latter is a tissue not created artificially but directly taken from a living organism.
Hannen first came across issues with human skin testing while she was starting her career at the Queen Mary University of London studying how steroids are made in the skin. She found that the pathway which is also involved in drug metabolism “shut down really fast.”
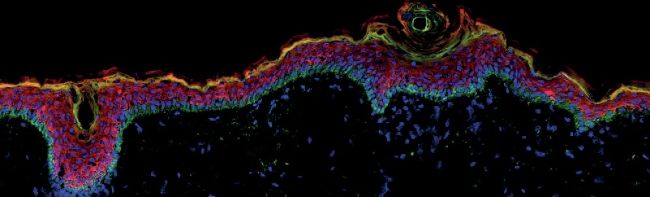
Image courtesy of Keratify using immunofluorescence
“I couldn’t do the experiments I wanted to do,” says Hannen. “Later I realised that this was actually a global issue across the whole of dermatology.”
She says that it was only in the past few years while establishing Keratify, a startup aiming to advance laboratory skin testing, that she realised the extent of the problem.
“We started to really look into test models and realised that these problems were widespread and not providing full information on the products, which was mind-blowing.”
Until Keratify, Hannen states, there was no preclinical skin model in existence that maintained viable cells and the skin barrier.
The biotech’s OneVivo skin culture device replicates the physiological environment of human skin. By doing this, it can maintain skin barrier properties and tissue function to high fidelity for more than five days.
“This means we can achieve new forms of skin testing that were never previously possible, as well as improving the accuracy of replicating human responses,” she explains.
THE PROBLEM WITH IN VITRO SKIN TESTING
Three-dimensional (3D) tissue models of the human skin formed the earliest examples of tissue engineering.
These models were developed as a tissue substitute for burns patients in clinics and by the cosmetics industry to replace animal models, which EU cosmetics companies were banned from testing on in 2013.
Regardless, Hannen explains, animal skin is a poor replacement for human skin, being as little as 59% accurate for some toxicology tests.
Today, a huge variety of 3D human skin models exist for many scientific endeavours. But research shows that current models are not able to fully maintain or replicate a competent skin barrier. “Many human 3D skin models are only 60-80% accurate for skin irritancy tests because of this and are five to 50 times more permeable than human skin,” says Hannen.
In some instances, the industry is under threat to reintroduce animal testing. “But the cosmetics and pharmaceutical industry doesn’t need animal models – it needs good human models,” Hannen adds. “We are working to ensure the right tests are in place so that this is not needed.”
Phenomenal innovation has been achieved in preclinical models where real skin is recreated with dermal fibroblasts, collagen gel and keratinocytes, but because of their permeability they can often overestimate the likelihood of irritation or other issues.
“These shortcomings and difficulties with in vitro assays have led to the cost of developing a new topical dermatology therapeutic being 50 times more than any other Phase I drug development,” says Hannen.
THE PROBLEM WITH EX VIVO SKIN TESTING
It makes sense that human skin tissue would and should be the only way to test cosmetic products and topical drugs, but the problem is that real human skin tends to deteriorate rapidly in an ex vivo (outside of the body or organism) environment. Meanwhile, regulatory guidelines stipulate that skin should be used within 24 hours for skin absorbance tests.
Skin is a highly complex tissue. It contains many different cell types and appendages, including immune cells, melanocytes, hair follicles and nerve fibres. Commonly used tissue culture incubators were designed to support cells and tissue from inside the body rather than human skin. A standard incubator is too warm and humid to sustain the skin barrier. The Franz diffusion cell was designed for skin permeation studies, as it can maintain the skin barrier, but it cannot keep the skin cells alive.
These concerns with both in vitro and ex vivo testing have meant that the cosmetics industry has hit a wall in terms of innovation, according to Hannen.
BARRIERS TO INNOVATION AND GOOD SKINCARE
In recent years numerous big pharma companies have sold off their entire dermatology portfolios, including Bayer and Allergan.
A lack of good models to test on and the exponential cost of running research are some of the reasons that the cosmetics and skincare industry’s new offerings are perhaps a lot less cuttingedge than we may have been led to believe.
“Instead of introducing novel compounds, the cosmetics industry has predominantly been adapting existing formulations,” explains Hannen.
“To innovate, the industry needs to know that its products are effective and safe on human skin, using reliable laboratory tests that fully replicate human responses.”
We often see compounds and ingredients in cosmetics that have first been approved through the medical sector, and then repurposed for cosmetics: “it kind of works backwards.” Retinoids are an example of this — first used as a medicine to treat acne with surprising anti-ageing benefits but also recognised as a vitamin and dietary supplement — the cosmetics industry has reduced concentrations to acceptable levels for consumer use.
Safety testing for cosmetics only needs to be done on the individual ingredients. There is no obligation to test in formulation, even though the act of formulation can sometimes change how chemicals behave, Hannen explains.
“Testing in formulation using 3D skin models is also often fundamentally difficult in the lab,” she continues. “Reviewing safety analysis of dermal therapeutics is easier than cosmetics due to stringent regulatory requirements for medicines. In contrast, the cosmetics industry is largely self-regulated.”
It is estimated that 10% of the population will experience some form of skin reaction from a cosmetic product in their lifetime and skin reactions are often underreported.
In recent years, the EU regulation Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), which assesses the chemistry and safety of compounds has carried out several big retractions in the industry — namely, preservatives and parabens — and notably, their gaze has turned to sunscreens.
“The controversy around certain chemicals in sunscreens is being reviewed across Europe and the implication of this could be huge,” says Hannen. “Considering that these chemicals are found in numerous formulations including make-up.”
Ensuring consumer safety is paramount, preventing irritation or sensitivity; however, Hannen says that the limitations of in vitro preclinical models also mean that many compounds that are perfectly safe for human skin use are being eliminated.
“The error rate is exceptionally high. Typically the industry would say that around 20% of certain chemistries are excluded but from our deep analysis, we’ve found that in some cases it’s more like 41%. To us, that feels like an unacceptable level.”
KERATIFY’S SOLUTION
Hannen states that up until Keratify’s solution, experiments looking at drug/compound permeation have had to be conducted entirely separately from experiments that look at functional responses e.g. toxicology and efficacy.
“This is because scientists didn’t have completely the right tools in place to maintain skin properly. But compounds typically have to pass through the skin barrier to exert a positive (useful) or negative (irritation, sensitization etc) effect. So doing these types of experiments separately without being able to fully consider skin barrier properties has led to fundamental problems across the board in advancing research and development for all forms of skin care — cosmetics, medical aesthetics, pharmaceuticals and transdermal delivery,” Hannen continues. The startup collects skin from routine plastic surgery with informed consent, approved by a research ethics committee.
“We only collect the skin that would otherwise go in the bin,” says Hannen. “Obtaining these tissues means we can create highly sophisticated analysis, ultimately ensuring safe and effective dermatology products can be created.”

Keratify’s newest OneVivo System
The OneVivo device then keeps the tissue healthy for longer than ever possible (a minimum of five days) by replicating the skin’s environment and keeping it temperature controlled. It is also connected to an electronic control unit and gas supply.
“We can then use the skin tissue to replicate the human response to a very high level of detail and can assess the surface of the skin biologically and visually. Previously in the lab, we could never do those things.”
The system is designed to pick up any chemical sensitivities in a quick cost-effective way, early in any formulation or product life cycle.
WHAT’S NEXT
Hannen says that one of the biggest challenges for her and the Keratify team now is “the re-education of a very educated market,” and admits that the problems with laboratory skin testing were not always known to her.
“The research was hard to find initially as it’s quite underreported, and we are just scratching the surface right now.”
In 2022 Hannen spoke at several prestigious international conferences about the industry’s shortcomings and was relieved to find her presentations well received.
“We recognise we are asking dermatology to reconsider the entire foundation of preclinical skin testing,” she says, “but dermatology researchers seem to be ready for the change as they have experienced the same issues I faced before founding Keratify.”
Keratify is now providing contract research to global pharma, consumer goods and cosmetic companies. They are also starting collaborations with academics across Europe.
The company is also working towards having its OneVivo technology built into regulatory guidelines, which can take years. Once that is achieved, there could be a sharp increase in new and exciting compounds coming to market.
ROS HANNEN
Ros completed her BBSRC-Unilever PhD studentship in dermatoendocrinology at the Blizard Institute, Queen Mary University of London (QMUL) in 2009. She won the British Skin Foundation grant award in 2016 to further her psoriasis research and was awarded a MedCity grant (2017, the highest-ranked grant) with Fourth State Medicine to assess the effectiveness of plasma technology for chronic wound healing. In 2018 Ros won the MedTech SuperConnector and QMUL Life Sciences Initiative awards to accelerate the commercial development of her research, and most recently an Innovate UK Smart grant. Ros founded Keratify in December 2018 to advance laboratory human skin testing.