CLINICAL
The safety and efficacy of fat-dissolving injectables for localised fat reduction
Carly Musleh talks through her study on the viability of injectables as a non-surgical option
In today’s society, weight and body image are deeply intertwined with cultural expectations and personal perceptions of health and beauty. The pressure to achieve and maintain a particular physique has intensified with the rise of social media, where idealised body shapes are often highlighted. This societal focus has led many individuals to explore various methods of weight management, including diet, exercise, and medical interventions. However, subcutaneous fat accumulation in specific body areas can be challenging to reduce through diet and exercise alone. As a result of social media, localised fat reduction through minimally invasive procedures has seen a surge in demand. One study found that 89.2% of adults used social media as a tool for weight loss information¹ and the term “fat-dissolving injections” generated a search volume of 14.8K on askthepublic.com.
Injectable fat-reduction techniques first gained attention in 2001 when Brazilian dermatologist Patricia Rittes reported using direct, transcutaneous injections of a phosphatidylcholine (PC) and sodium deoxycholate (DC)-based product Lipostabil to reduce infraorbital fat.2 In the gastrointestinal tract, deoxycholate acid (DCA) functions primarily to emulsify fats, allowing better absorption of dietary lipids. It is produced by the bacterial breakdown of cholic acid in the colon and recirculated through the enterohepatic circulation.3 DCA is used as the active/drug in an injectable form (e.g., in treatments like Belkyra) acting as an ionic detergent, causing adipocytolysis, by disrupting the integrity of the adipocyte (fat cell) membrane and dissolving hydrophobic molecules. This releases the cellular components into the surrounding area, allowing the body’s immune system to metabolise and remove the fat.
Fat-dissolving injections which include these ingredients are classified as medical devices since they modify the body’s physical condition without triggering a pharmacological or metabolic response, which would otherwise categorise them as medicines. Medical devices placed on the UK market must have a UKCA (UK Conformity Assessed) mark, or a CE mark (used in the European Union). These marks indicate that the product complies with applicable General Safety and Performance Requirements (GSPR), as set by UK or EU regulations.4 It is the manufacturer’s responsibility to ensure the product meets all regulatory standards and affix the UKCA or CE marking to indicate compliance. This process involves assessing the product for safety, performance, and quality.
Some well-known fat-dissolving injection brands which carry these marks are Aqualyx, Belkyra, Desoface and Desobody. Lemon Bottle (LB) has recently become a popular brand of fat-dissolving injectables that has gained widespread attention due to social media highlighting its alleged rapid and visible results.
However, despite its rising use, the safety, efficacy, and long-term effects of such treatments remain areas of concern. There is a significant lack of comprehensive clinical research on the long-term effects and mechanisms of products like LB. Most available data are anecdotal or based on short-term studies that asses immediate results without longterm follow-up. Furthermore, many studies focus on those classified as medical devices containing deoxycholic acid, while little is known specifically about the proprietary formulations in products like LB. A user’s guide for LB claims that unlike fat-dissolving injectables which include DCA or PC, the treatment’s effectiveness is due to three key ingredients: riboflavin (B2), bromelain, and lecithin.
The lack of robust, peer-reviewed clinical trials contributes to uncertainty about optimal dosage, treatment protocols and individual risk factors that may predispose patients to complications. These knowledge gaps pose a challenge for both practitioners and consumers, leading to inconsistent results and safety concerns. In March 2024, Swissmedic declared LB lipolysis solution is not approved for distribution in Switzerland and must not be sold or used.5
The primary objective of this study is to evaluate the safety and efficacy of two LB formulations using analytical and biological techniques. Specifically, the study aims to assess the formulation’s chemical profile through LCMS, NMR, and UV-Vis spectroscopy, while determining its cytotoxicity through in vitro cytotoxicity tests. Additionally, the lipolytic activity of the LB will be evaluated by injecting it into bacon fat and observing the effects on fat breakdown through both microscopic and macroscopic observations. These combined analyses will provide a comprehensive understanding of the formulation’s biochemical properties and potential therapeutic or cosmetic applications.
METHODS AND MATERIALS
Chemicals and reagents
• LB fat-dissolving injection (three commercially obtained from different suppliers online). As per manufacturer the formulation is as follows: LB is composed of water, ananas sativus (pineapple) fruit extract (5%), pentylene glycol (3%), bromelain (physiotherapeutic protein stem)(1%), sodium chloride (0.9%), lecithin (0.1%), centalla asiatica extract (gotu kola) (0.1%), salvia miltiorrhiza root extract (0.05%), chamomilla recutita (matricaria) extract (0.05%), scutellaria baicalensis root extract (0.05%), riboflavin (vitamin B2 (0.01%)
• Breast cancer cell line: MCF-7 cells were chosen for cytotoxicity evaluation
• Unsmoked bacon fat: Used as an ex-vivo model for fat tissue
• Solvents and standards: Analytical-grade methanol, deuterated chloroform for NMR, 1% glacial acetic acid solution and other chemicals were purchased from commercial suppliers
• Pineapple extract: Fresh pineapple fruit was purchased from a supermarket
• Riboflavin (B2): sigma aldrich (Gillingham, UK)
• 30-gauge needle and syringe: Medical grade supplies
• pH test: Hanna digital pH meter (calibrated)
• Media: Solid - Solid horse blood agar (for optimal culture of gram-positive bacteria), CLED chromogenic agar (for optimal culture of gram-negative bacteria), nutrient agar, tryptone soy agar, malt agar (for optimal culture of yeast and fungi) Liquid -Nutrient broth, tryptone soy broth.
Centrifugation and precipitation of pineapple extract for LC-MS
To extract the supernatant from fresh pineapple for analysis centrifugation was used. Solid pieces of fruit and stem were crushed using a pestle and mortar. The liquid was placed into the centrifuge for 15 minutes at 14000 RPM. The supernatant was then filtered to help isolate specific compounds before running the LC-MS.
Preparation of riboflavin (B2) solution
A 0.01 g sample of riboflavin (B2) was weighed and dissolved in 10 mL of deionised water to prepare a 1 mg/mL B2 solution. The mixture was subjected to sonification to ensure complete dissolution and homogeneity.
The prepared riboflavin solution was analysed to obtain a reference profile for comparison. To investigate the presence of riboflavin or similar compounds in the LB solution, 1 drop (~50 µL) of the B2 solution was spiked into a fresh vial of the lemon bottle sample. The spiked solution was then analysed by LC-MS, and the resulting chromatograms were compared to identify any shifts or overlapping peaks.
Liquid chromatography-mass spectrometry (LC-MS)
Gradient HPLC was performed on an integrated Agilent® 1290 UHPLC system. The flow rate was 0.6 mL/min and the injection volume was 10 µL. A post-injection needle wash was included in the method to rinse for 3 secs with methanol. The column temperature was automatically regulated at 35 °C. Mobile phase A consisted of 0.1 % v/v formic acid in 18.2 mΩ-cm2 water. Mobile phase B was 0.1 % v/v formic acid in methanol. Separation was performed on an ACE C18 column (250 mm x 4.6 mm, 5 µm), (advanced chromatography technologies, Scotland, UK). Primary detection was by UV at 250 nm using a variable wavelength detector. Secondary detection was performed with an Agilent 6120 Q-MS operating in negative mode with electrospray ionisation (ESI). The system was programmed to scan the mass range from 100 to 1500 m/z. LC/MS operation and data analysis were performed using Agilent LC/MSD ChemStation software, version B.04.03 (Agilent Technologies, Delaware, USA).
Nuclear magnetic resonance (NMR)
To further explore the molecular profile of the LB solution, nuclear magnetic resonance (NMR) spectroscopy was performed. All 1H NMR data were recorded on a Bruker AVANCE III NMR spectrometer at 500.13 at 300 K by using a 5-mm BBI inverse probe equipped with a z-gradient coil. Data were acquired using the Topspin software.
Spectra were acquired by using a pre-saturation pulse sequence and further suppression of baseline artefacts was achieved by carrying out incorporating a 1D NOESY sequence. A relaxation delay of 2.4 secs; acquisition time 1.6 secs; spectral window 10,000 Hz, 32,768 data points and 1024 scans were used to collect the data. A 0.5 Hz line broadening procedure was applied before Fourier transformation.
Ultraviolet-visible spectrophotometer (UV-Vis)
Sample preparation: Deionised water was used as the blank to zero the instrument and account for any baseline absorbance. A small volume (e.g., 1 mL) of LB solution was diluted with deionised water to ensure the absorbance values were within the detection limits of the instrument. Both samples were transferred into clean quartz cuvettes with a 1 cm path length.
The ATi Unicam UV/VIS was calibrated using the blank sample prior to the analysis. The absorbance spectrum for the LB solution was recorded between 200 nm and 800 nm, which covers the UV and visible range. Absorbance values were measured at key wavelengths associated with fat-dissolving components, including the yellow spectrum range (420-430 nm), to investigate any colour-related compounds.
Cytotoxicity test on breast cancer cells (MCF-7)
General cell culture: Cell culture experiment was performed in a humidified 5% (v/v) CO2 air atmosphere at 37°C in a complete medium. MCF-7 was cultured in complete media RPMI, supplemented with 10% FBS, L-glutamine (2.5 mM) and Sodium pyruvate (1 mM) for optimal cell growth. Cell line was routinely grown in T-75 flask Sigma-Aldrich (Gillingham, UK). The cells were split twice a week after reaching 80% confluency using sterile PBS pH 7.4 for washing and trypsin-EDTA for trypsinisation.
Cytotoxicity: MCF-7, was seeded in 96-well plates (CLS3788, Sigma-Aldrich, UK) at a density of 10,000 cells per well. Then incubated overnight at 37℃ before treatment. Afterwards, cells were washed with fresh media and then treated with different drug groups. In addition to the untreated cells as a negative control. After treatments, cells were incubated with the drugs for 48h, then the cells were ready to be assayed.
Cell Proliferation Assay – MTS: MTS assay was carried out according to the manufacturer’s protocol. Briefly, cells were washed with the media twice, then 20 μl of MTS solution was added to each well (treated/or untreated). In addition to a blank well and negative control for background subtraction and calculation of cell viability, the 96-well plates were incubated for 1–4 hr in 5% CO2 at 37℃. Afterwards, the plated wells were measured using the microplate reader (SpectraMax i3x, SoftMax Pro 7.0 software. Molecular Devices, UK) at 490 nm.
Lipolysis test on bacon fat
Unsmoked thinly sliced bacon was selected as a fat tissue analogue. For each test, one piece of bacon was placed on a sterile surface. The bacon was chosen for its high-fat content and fat profile, providing a uniform surface to mimic subcutaneous fat layers. For comparative analysis distilled water was used as a negative control and 1% glacialacetic acid solution as the positive control. A 30-gauge needle was used for all injections to minimise tissue disruption.
A total of eight injection points were marked evenly across each bacon slice. Each injection point received 0.1 ml of the respective solution, resulting in a total volume of 0.8 ml per bacon slice. The needle was inserted perpendicular to the surface of the bacon slice, and the solution was injected slowly to ensure even distribution across the fat layer.
Each solution was injected into the bacon slices using a consistent procedure to assess their potential fatdissolving properties. Care was taken to avoid leakage of the injected solution from the bacon tissue.
After injection, each bacon slice was left at room temperature for 60 minutes to observe any visible changes, such as fat dissolution, liquid accumulation, or tissue breakdown.
pH test of LB
Each vial of LB was carefully opened under sterile conditions. Prior to testing, the digital pH meter was calibrated using standard buffer solutions to ensure accurate readings. The probe was rinsed with distilled water and dried between measurements.
The pH probe was inserted directly into the first LB vial, and the pH reading was recorded once stabilised. The same procedure was repeated for the second vial. After each measurement, the pH probe was rinsed thoroughly with distilled water to prevent cross-contamination. The pH values for both vials were recorded and compared against the standard pH range for injectable solutions.
Bacterial culture analysis
As a final test, to prevent environmental contamination, an unopened vial of LB was handled within a sterile safety cabinet. A 1 µL aliquot of the LB solution was cultured using the streak plate method on various solid agar media to optimise bacterial identification. Additionally, 10 µL of the LB solution was inoculated into the liquid media to enhance bacterial enrichment in cases where bacterial presence was minimal.
All cultures were prepared in duplicate and incubated at 36°C to promote optimal bacterial growth and at 25°C to support the growth of yeast and fungi.
RESULTS
LC-MS analysis: LC-MS analysis was performed using a scouting gradient method in positive mode with a scan range of 100-1500 m/z in order to detect as many ions as possible, including protein fragments which might be expected for a sample containing bromelain. LB samples named LW and FW appeared to be identical under these test conditions (Figure 1), both containing a peak at 6.6 minutes which appeared to be peptide-related based on the MS fragment ion patterns (Figure 2).
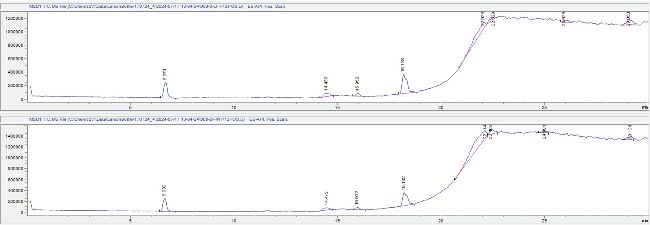
Figure 1: LCMS total ion chromatograms for LF sample (top) and FW (bottom), showing compound peaks at 6.6 min, 14.4 min, 11.6 min, 15.9 min and 18.1 min. Spectra from these peaks are shown in the appendix.
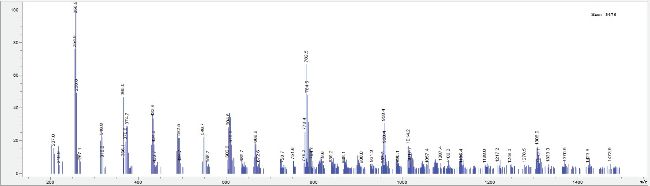
Figure 2: Extracted spectrum from peak eluting at 6.6. minutes in the LF sample.
In the absence of an analytical standard of bromelain, raw pineapple juice was used for comparison following a similar methodology for the separation and detection of bromelain as reported in literature.6 Compounds in the pineapple juice were observed to elute at the same time as the suspected bromelain peak in LB, but the LCMS data was inconclusive towards confirming the identity due to the complex nature of the spectra and the limitation of the single quadrupole analysis. The UV peak intensity observed in Figure 3 suggests that the concentration of the suspected bromelain in LB is significantly lower than that occurring in the pineapple juice. This may be due to the reported limited shelf life of bromelain which has self-digesting properties and a propensity to undergo reducing sugar reactions at room temperature.7
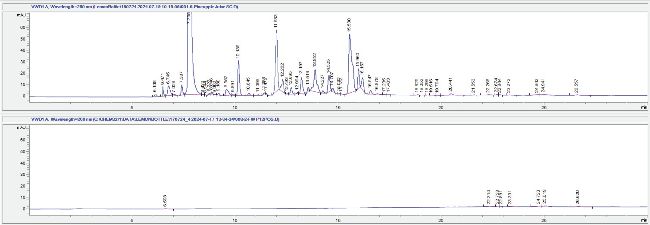
Figure 3: LCUV chromatograms (260 nm) for pineapple juice extract (top) and FW (bottom) presented in the same scale.
The presence of riboflavin, a listed ingredient, was also investigated by LCMS using an analytical standard of this molecule as a reference standard. The reference riboflavin eluted at 15.07 min with a strong UV response at 260 nm and a corresponding peak in the mass spectrometer relating to the [M+H]+ ion with a m/z of 377. This peak is absent in the LB sample LCUV trace as shown in Figure 4, and no related ion could be detected in the ion chromatogram resulting from a highly sensitive selective ion monitoring experiment. Taken together, this data indicates that if any riboflavin is present in the LB sample it is at such a low concentration that our analysis could not detect it.
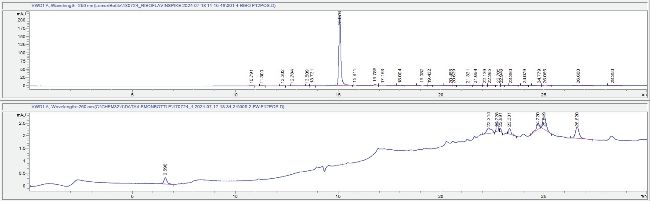
Figure 4: LCUV chromatograms (260 nm) for riboflavin standard (top) and FW (bottom) each at full scale.
NMR Spectroscopy
A water suppression pulse sequence was used to determine the 1H spectrum for the components present in the sample. Initial analysis at 32 scans showed no spectral peaks other than those belonging to the listed ingredient pentylene glycol and water (suppressed). To increase sensitivity, the number of scans was increased to 1024 which revealed unidentified small spectral peaks from 3-3.8 ppm and 7.5-8.5 ppm, suggesting the presence of some aromatic molecules at low concentrations (Figure 5). These aromatic signals did not match the literature spectral data acquired for riboflavin under similar conditions,8 indicating that this molecule was not present at detectable concentration.
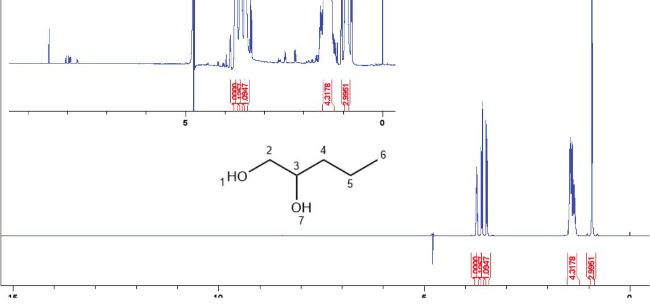
Figure 5: 1H NMR spectrum of LB sample with 10% D2O (0.05 mM TSP), acquired using water pre-saturation pulse sequence n=1024 scans. Figure inset showing unidentified compound signals near S/N threshold. Main spectral peaks relate to pentylene glycol (inset): 0.9 ppm = protons at position 6, 1.2 ppm = protons at positions 4 and 5 overlapped, 3.5 ppm and 3.65 ppm = diastereotopic protons at position 2, 3.8 ppm = proton at position 3.
UV-Vis analysis
An absorbance peak was detected using the UV spectrometer; however, no corresponding peak was observed in the HPLC analysis at the same wavelength. This discrepancy suggests that the UV-absorbing compound is either unretained or fully unretained on the column. Given the hydrophobic nature of the stationary phase used, it is more likely that the former may have occurred. Subsequent analysis utilising hydrophilic interaction chromatography is recommended.
Cytotoxicity test on MCF-7 cells
The results showed that both fat dissolving solution samples didn’t exert any cell inhibition on MCF-7 cancer cell line. On the contrary one of the samples showed a slight increase in cell viability (Figure 6). The results support the hypothesis that the LB product doesn’t contain any components that exert anticancer effects or cytotoxicity to breast cancer cell lines. This indicated that at therapeutic concentrations used for fat dissolution (1-10 µg/mL), the formulation is likely safe for human cells.
However, higher concentrations may cause cellular damage, highlighting the importance of dosage control. Figure 6. Cell viability in MCF-7 cells treated with the fat-dissolving commercial product. The graph shows a comparison between the control group (untreated cells), LB and PBS. Significance level is presented as (ns; no significance), and (**; P ≤ 0.01).
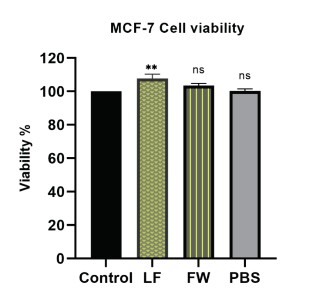
Figure 6: Cell viability in MCF-7cells treated with fat dissolving commercial product (LB). The graph shows a comparison between control group (untreated cells), LB and PBS. Significance level is presented as (ns; no significance), and (**; P ≤ 0.01).
Lipolysis test on bacon fat
In the lipolysis test, neither of the bacon slices injected with the two LB solutions exhibited any visible reduction in fat mass at the injection sites after 60 minutes, based on macroscopic observations. Microscopically, there was no disruption in fat cell structure and no evidence of fat globule shrinkage or tissue degradation. As expected, the distilled water showed no fat dissolution, confirming its role as a negative control. In contrast, the 1% glacial acetic acid, a known fat-dissolving agent, caused significant fat breakdown, validating its use as a positive control in this study. The injection volume per point (0.1 ml) was deliberately lower than the manufacturer’s recommended dose (1.5–2 ml per injection point), given the thinner nature of the bacon slice compared to human adipose tissue.
pH of LB
The pH measurements for both LB vials were found to be 6.0, which falls within the standard pH range for common subcutaneous injectable solutions. This indicates that the LB solution maintains a suitable pH for injections.
Bacterial culture analysis
No bacterial, yeast, or fungal growth was observed on any of the cultures after 24 and 48 hours of incubation.
DISCUSSION
Despite utilising advanced techniques such as LC-MS, NMR, UVVis, and cytotoxicity assays, the findings raise significant concerns about the product’s claimed active ingredients and its overall efficacy. The analysis failed to detect any of the advertised active components, including bromelain, riboflavin, or lecithin, in either of the two LB vials, which were purchased from different online suppliers. Pentylene glycol was the only identifiable compound via NMR, and water, although excluded from analysis due to its high volume, was the major constituent.
One major safety concern arising from these findings is the potential harm of injecting large quantities of water. For the submandibular area (double chin), LB recommends injecting 10–20 cc (left and right sides). Given that 1 cc is equivalent to 1mL, and each vial contains 10 mL of LB solution, this suggests the use of 2–4 vials (20–40 mL) per treatment, repeated as often as weekly. While water is generally safe for consumption and external use, injecting it into the subcutaneous layer poses significant risks. Large volumes of water or water-based solutions can cause tissue damage, inflammation, and create dangerous imbalances in local osmotic conditions.
Additionally, water alone lacks any active fat-dissolving properties, and its introduction into fat tissue may lead to swelling, infection, or even death of skin tissue, especially if not administered properly.
Numerous viral videos online claim that LB effectively breaks down fat when injected into bacon fat. These demonstrations, conducted by both medical and non-medical individuals, show a comparison between treated and untreated fat, with the claim that LB causes visible fat reduction. However, our controlled lipolysis test failed to support these claims. Neither of the LB vials exhibited any lipolytic effect on the bacon fat tissue, further corroborating our findings that the products contained no active ingredients capable of enzymatic fat breakdown. The absence of any fat-dissolving activity in these tests suggests that the product’s advertised effects are unfounded, at least in this model. However, further studies are necessary to assess whether similar results would be observed in human adipose tissue, especially in thicker fat deposits. Although it is an injectable formulation intended for fat reduction, it does not meet the definition of a cosmetic under Article 2 of Regulation (EC) No 1223/2009, which pertains to products applied to external body surfaces. Swissmedic, the regulatory authority in Switzerland, has explicitly stated that injectable products fall under the law governing medicinal products due to their method of administration.
Swissmedic has declared that LB is not marketable in Switzerland and should not be sold or used, underscoring the need for proper regulation of injectable treatments.
In contrast, the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) does not classify LB as a medical product, as it does not contain recognised pharmaceutical ingredients such as phosphatidylcholine or deoxycholic acid, nor does it make explicit medical claims. This regulatory gap leaves the product under cosmetic regulations, meaning it does not require a UKCA or CE mark for sale in the UK, additionally, the lack of regulation on who can administer injections poses significant safety risks. Cases of skin necrosis due to superficial injections by unqualified practitioners have already been reported, highlighting the dangers associated with the product’s widespread and unregulated use.
The ease of purchasing LB online is another critical issue. Consumers can buy the product without any verification of its authenticity or assessment of whether they are qualified to use it. The lack of control over who can purchase and inject the product opens the door to misuse, improper administration, and counterfeit products, all of which can have serious health consequences. Additionally, the absence of any certification or quality control increases the risk of receiving products that contain harmful contaminants or incorrect formulations.
FUTURE CONSIDERATIONS
Given the concerning results of this study, further research is necessary to fully evaluate the safety and efficacy of LB. Future studies should focus on in vivo testing to better understand the systemic and long-term effects of the LB fat-dissolving solution.
These studies could assess how the product behaves in living tissue and whether it induces any adverse effects.
Although no bacterial, yeast, or fungal growth was observed on any of the cultures, it would be advantageous to repeat the experiment under varied environmental conditions, and with an extended incubation period. This would help account for microorganisms that may require specific conditions or longer time frames. This is particularly significant given the widespread availability of the product online, where limited regulatory oversight increases the risk of unsafe or counterfeit formulations. Comparative studies comparing LB with other fat-dissolving injectables on the market could provide valuable insights into its relative efficacy and safety. Such comparisons could also help to clarify whether any of the claims made about LB hold up under scientific scrutiny.
Lastly, additional cytotoxicity testing on other relevant cell lines, such as fibroblasts or adipocytes, would provide more comprehensive safety data. This could help assess the potential impact of LB on different types of cells in the body.
In conclusion, the findings of this study question the efficacy of LB as a fat-dissolving injectable and highlight significant safety concerns, especially considering the lack of regulation and oversight in its distribution and use. Further research is crucial to provide more conclusive evidence and to safeguard consumers from potential risks.
CARLY MUSLEH
Carly Musleh is a cosmetic chemist, academic tutor, and published scientific communicator. With 18 years of experience in the beauty industry, she specialises in translating complex scientific information into accessible, evidence-based insights, challenging misinformation and decoding marketing jargon. Musleh creates educational content that has been featured by several international news outlets, empowering both professionals and consumers with science-backed knowledge. Dr Stephen Childs and Dr Tia Attia from the University of Sunderland also contributed to investigation and writing.
REFERENCES
1. Alzaben AS, Alzaidy KI, Alghamdi MA, et al. The use of social media to search for weight reduction information: Assessment of the perception among a sample of Saudi adults. Digit Health. 2022;8:20552076221136939. Published 2022 Nov 4. doi:10.1177/20552076221136939
2. Rittes PG. The use of phosphatidylcholine for correction of lower lid bulging due to prominent fat pads. Dermatol Surg. 2001;27:391-392.
3. Ai-jin Xie, Chu-tian Mai, Yi-Zhun Zhu, et al. Bile acids as regulatory molecules and potential targets in metabolic diseases, Life Sciences, Volume 287, 2021, https://doi.org/10.1016/j.lfs.2021.120152.
4. Medicines and Healthcare products Regulatory Agency. Regulating Medical Devices in the UK [Internet]. GOV.UK. 2020. Available from: https://www.gov.uk/guidance/regulating-medical-devices-in-the-uk
5. Copyright Swissmedic 2019. Swissmedic issues warning about the illegal medicinal product “Lemon Bottle” lipolysis solution [Internet]. Swissmedic.ch.2024. Available from: https://www.swissmedic.ch/swissmedic/en/home/humanarzneimittel/marketsurveillance/medicinal-products-from-the-internet/warnings-related-to-medicinalproducts-from-the-internet/warnung-lemon-bottle.html
6. Reddy Bm, Ashok Reddy Br, Naidu Ut, et al. A Simple RP-HPLC Method Development and Verification of The Dissolution of Bromelain-A Complex Mixture of Proteolytic Enzymes, In Delayed-Release Tablets. International Journal of Pharmacy and Pharmaceutical Sciences [Internet]. 2024 Jan 1 [cited 2025 Jan 11];37–44. Available from: https://www.aurigeneservices.com/sites/default/files/202404/Ashok_article_IJPPS_Bromelain.pdf
7. Gross P, Seelert H, Meiser P, Müller R. Characterization of bromelain indicates a molar excess of inhibitor vs. enzyme molecules, a Jacalin-like lectin and Maillard reaction products. Journal of Pharmaceutical and Biomedical Analysis. 2020Mar;181:113075.
8. Human Metabolome Database: 1H NMR Spectrum (1D, 500 MHz, H2O, experimental) (HMDB0000244) [Internet]. Hmdb.ca. 2025 [cited 2025 Jan 11]. Available from: https://hmdb.ca/spectra/nmr_one_d/1268#conditions Appendix: Mass spectra
