RESTYLANE KYSSE COMMERCIAL FEATURE
Beautiful, plump, natural-looking lips
Looking at hyaluronic acids and how Restylane Kysse is designed differently
If you think hyaluronic acid (HA) injectables are all pretty much the same, think again. They can vary hugely, from those delivering rudimentary volumisation to others creating a beautifully natural look. The Restylane range has been specifically developed to help aesthetic practitioners make the very most of their skills, for highly customised results that meet patients’ individual needs. This includes Restylane Kysse, specifically designed to deliver smooth, plump, natural-looking lips.1-4 Here we consider the key benefits of Restylane Kysse and what differentiates it from other HA injectables in terms of design, natural-looking results and safety profile.
LONG-LASTING RESULTS WITH LESS PRODUCT†
Restylane Kysse delivers up to 12 months of optimal performance with less product than comparable products.5,6 In a head-to-head clinical study, Restylane Kysse achieved desired lip volume with 20% less product than a competitor product:5
OPTIMUM BALANCE OF FLEXIBILITY AND SUPPORT
Restylane Kysse has specific gel properties based on Optimal Balance Technology (OBT).7,8 This combines high xStrain to facilitate movement1,8* with a soft, flexible gel texture to distribute evenly for balanced volume and a natural look.3,4,9
HIGH PATIENT AND PARTNER SATISFACTION
Restylane Kysse offers high satisfaction for both patients and their partners:3,6
• 100% of patients were happy with their lip style and reported aesthetic imoprovement3†
• 96% of patients agreed their lips looked natural3**
• > 98% of patients were satisfied with the kissability of their lips3**
• 90% of partners liked the appearance of their partners lips3**
• 73% of partners agreed their partner’s lips had a more kissable and natural feel.3**§
WELL ESTABLISHED SAFETY PROFILE
Restylane Kysse has a well-established safety profile, demonstrated across a diverse range of patient groups, including those with different skin types, colours and tones.1,4,6 Swelling, tenderness, bruising, pain and redness at the injection site are the most commonly observed side effects, but resolution is typically spontaneous within a week after injection.2 Please refer to Instructions for Use for full product information.
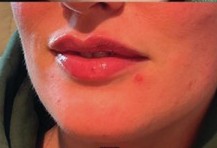
Treated with Restylane Kysse in the lips by Dr Zoya Awan. Permission sought to use patient’s images.
Before and after images for illustrative purposes only and individual results may vary. All rights reserved.
DR ZOYA AWAN
“Throughout my years of practice, I’ve catered to patients of diverse age groups, each with unique needs when it comes to lip enhancement. In my opinion, with fleeting trends where dramatic transformations are common, natural-looking lips never go out of style. My experience with Restylane Kysse filler has been truly remarkable. It’s a versatile solution that delivers a natural look whether they’re seeking to enhance youthful fullness or restore subtle volume.”
GETTING THE MOST FROM THE RESTYLANE RANGE
The smooth, plump, natural-looking lips achieved with Restylane Kysse can be complemented with other Galderma products for a Holistic Individualised Treatment (HIT).
To learn more, speak with your Galderma sales representative or contact sales.UK@galderma.com.
Dr Zoya Awan is a key opinion leader for Galderma. This advertorial was created and funded by Galderma.
GB-RES-2400136 DOP August 2024
† Compared with Juvéderm® Volbella5
* Strain is an index of gel flexibility (how much strain a gel can withstand and still be reversible)
** As assessed by partners in the Galderma KISSABILITY Questionnaire, which was provided at Week 4 and returned at Week 63
§ Compared with baseline3
^ Based on a global survey with 471 HCPs across US, Brazil, Germany and Thailand
REFERENCES
1. Galderma. Data on File. MA-43049.
2. Restylane® Kysse™ EU IFU.
3. Bertucci V et al. J Cosmet Dermatol 2021; 20(5): 1499-1504.
4. Bertucci V et al. J Drugs Dermatol 2021; 20: 402-408.
5. Weiss R et al. Dermatol Surg 2021;47(4):527–32.
6. Hilton S et al. Dermatol Surg 2018;44(2):261–9.
7. Rogerio V et al. J Stomatol Oral Maxillofac Surg. 2022;123(4):440–7.
8. Öhrlund Å. J Cosmet Dermatol Sci Applic. 2018; 8(2): 47-54.
9. Lundgren B et al. J Drugs Dermatol 2018; 17(9): 982-986.
Adverse events should be reported. For the UK, Reporting forms and information can be found at mhra.gov.uk/yellowcard or search for Yellow Card in the Google Play or Apple App Store.
For Ireland, Suspected adverse events can be reported via HPRA Pharmacovigilance, Website: hpra.ie; Adverse events should also be reported to Galderma (UK) Ltd, Email: medinfo.uk@galderma.com Tel: +44 (0) 300 3035674