INFECTION CONTROL
Scrubbing up
The second part of ACE Group’s new guidelines on infection control in aesthetic medicine
In last month’s (February 2021) issue of Aesthetic Medicine, we published the first part of the new guidelines on infection control in aesthetic medicine from ACE Group World (Aesthetic Complications Experts). In this second part, aesthetic nurse Helena Collier, who authored the guidelines, covers best practice hand hygiene and glove usage.
HAND HYGIENE AND GLOVE USAGE
Hand hygiene
Handwashing is considered the most important barrier to cross-infection. There is now undisputed evidence that strict adherence to hand hygiene reduces the risk of infections.³⁵ The hands are colonised by two types of microbes: the permanent resident flora that consists of micro-organisms living under the cells of the stratum corneum and the transient flora which colonise the superficial layers of the skin35 Transient flora are not present on the skin at all times; they may transfer during patient contact and by the touching of inanimate surfaces and are more susceptible to removal from the skin with handwashing.
Although handwashing in healthcare has improved in recent years, good practice is not universal. Research continues to show low compliance rates among healthcare workers.³⁵ Increasing healthcare workers compliance with hand hygiene remains a global challenge27 Hibiscrub is an antimicrobial skin cleanser containing 4% chlorhexidine gluconate and is widely used in both primary and secondary care. Hibiscrub will inactivate micro-organisms present on the hands to prevent transmission of microbes. This is achieved by the active ingredient, chlorhexidine gluconate, binding to the skin to form an effective barrier that will not only kill bacteria but continue to give residual protection for several hours.
NICE guidelines advise that when delivering direct patient care, wrist and hand jewellery are removed, fingernails are short, clean and free of nail polish and any cuts or abrasions covered36 Moreover, the bare below the elbow rule must be adhered to, appropriate glove usage implemented, and a single use disposable apron adorned as part of personal protection equipment when delivering direct medical aesthetic care.
Glove usage
The decision to wear gloves is based around an assessment of the risk of contact with blood, body fluids, secretions and/ or excretions, non-intact skin, mucous membranes, chemicals, or hazardous drugs37 Gloves are worn to protect both the HCP and the patient. Table 4 provides a summary of glove usage/removal.
Table 4: Summary of the indications for glove usage and for glove remova38
GLOVES ON
Before a sterile procedure.
When anticipating contact with blood or another body fluid regardless of the existence of sterile conditions and including contact with non-intact skin and mucous membranes.
Contact with a patient (and his/her immediate surroundings) during contact precautions.
GLOVES OFF
As soon as gloves are damaged or nonintegrity suspected.
When contact with blood, another body fluid, non-intact skin and mucous membrane has occurred and has ended.
When contact with some single patient and his/her surroundings, or a contaminated body site on a patient has ended.
When there is an indication for hand hygiene.
It has long been recognised that the wearing of gloves reduces the transmission of micro-organisms and contaminated matter via the healthcare worker’s hands, however, there are growing concerns that the wearing of gloves is becoming a substitute for effective hand hygiene39 Gloves do not eliminate contamination of hands and become a source of infection risk to patients if not changed appropriately between each procedure and patient contact. Several studies have identified that the compliance level of hand washing significantly reduces when gloves are w39,40
The prolonged use of gloves in the absence of considering the need to perform hand washing can result in the transmission of microbes. Moreover, there are reports of healthcare professionals decontaminating gloves with soap and water or an alcoholbased hand rub as a means of infection control between patients or procedures39
Disposable gloves are single use medical devices and in accordance with health and safety requirements are considered personal protective equipment37 Pathogens can contaminate gloved hands by means of defects in the glove material or during removal of the gloves. Hand washing with an appropriate anti-microbial solution such as Hibiscrub is essential practice before the application and the removal of gloves. No glove material provides absolute protection against all potential risks in the clinical setting.
Evaluation of glove types
DISPOSABLE VINYL GLOVES
Low tensile strength, rigid and inflexible and are prone to splitting.³7
Increased permeability to blood borne viruses – unsuitable for any procedure where there is a risk of contact with blood or other body fluids.³7
Leakage rates of up to 63% reported.³7
Vinyl gloves, polythene gloves or gloves made from copolymer material should not be used for clinical purposes56
DISPOSABLE NITRILE GLOVES (STERILE AND NON-STERILE)
Reliable barrier against viral and blood borne pathogens37
Good protection against microbial contamination37
Highly resistant to tears or punctures37
Brightly coloured making gloves easy to identify.
Suitable alternative to latex gloves when sensitisation/allergy has been identified37
Non--sterile disposable nitrile gloves are suitable for examination purposes and procedures that do not breach the skin barrier27
Sterile nitrile gloves, refer to heading: Sterile surgical gloves.
“The hands are colonised by two types of microbes: the permanent resident flora living under the cells of the stratum corneum and the transient flora which colonise the superficial layers of the skin”
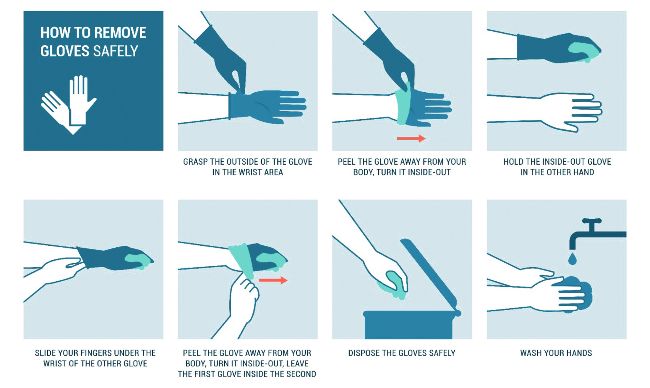
Figure 1: The correct removal of contaminated gloves.
DISPOSABLE LATEX GLOVES (STERILE AND NON-STERILE)
Give the best fit, best dexterity, and most comfort due to high elasticity and strength.³7
High degree of tactile sensitivity37 Excellent microbial barrier protection.
Found superior to nitrile in simulated needle stick injury study.⁴¹
Increasing prevalence of latex allergy has led to reduction in use37
Nitrile gloves are a safer alternative.
Sterile latex gloves, refer to heading: Sterile surgical gloves.
Sterile surgical gloves
Sterile gloves should be worn for surgical procedures, for invasive procedures and for the insertion of an invasive device37
The injection of dermal filler should be considered an invasive procedure and the insertion of a device, both requiring the execution of an aseptic technique.
All medical aesthetic procedures that breach the skin’s integrity should merit the application of sterile gloves. It is important to carefully don sterile gloves to protect the patient, but it is as equally important to remove gloves correctly to reduce the risk of contamination to the practitioner.
Aseptic technique
Aseptic technique can be defined as a healthcare procedure designed to minimise the risks of exposing a patient to pathogenic micro-organisms during simple and complex procedures36 The application of aseptic technique is central to reducing healthcare associated infections. It is presumed that healthcare professionals reading this guidance are experienced in the execution of aseptic technique thus precluding the need to give a step-by-step account within this guidance. Aseptic technique must be applied when administering dermal filler.
Dermal filler-associated infections
Infection rates after the injection of dermal filler are reported in the literature as low32 However, the author postulates that there is a growing body of evidence to support that complications once thought to be of an immunogenic nature are in fact of an infectious nature. This is compounded by the reluctance of practitioners to report complications gives rise to the likelihood of inaccurate data on infection rates associated with dermal fillers27
There is no universal reporting system in place to collate accurate data regarding infection rates. The literature supports that the most common infections arise when skin contaminants infiltrate the site of injection at the time of injection32 (Refer to the Aesthetic Complications Guidelines on Acute Infection).
This would suggest that inadequate skin disinfection prior to injecting plays a significant role in this process. This is further supported in the work of Wagner who also reports microbes being introduced through direct inoculation during injection or by the haematologic spread of a systemic infection31 Clinical evidence supports that if active infections such as sinusitis, periodontal disease, ear, nose, or throat infections are present at the time of injecting dermal filler, there is a risk the infection could subsequently invade the implanted filler material inducing a biofilm. It is therefore imperative that patients are well and infection free at the time of injecting.
Furthermore, there is a substantial volume of evidence in the literature to support that infections that originate in the sinuses, face, ears or oral cavity have the potential to drain into the cavernous sinus with grave life-threatening consequences, dermal filler associated infection in these areas are no except44,45 Cavernous sinus Infection can lead to the formation of multiple abscesses and result in neurological and/or ophthalmological damage.
A septic cavernous sinus thrombosis has the potential to occlude the retinal artery leading to permanent iatrogenic blindness44 This strongly supports why infection control within aesthetic medicine must be taken very seriously; because the consequences of inaction could prove to be catastrophic or indeed, fatal.
Acute skin infection
Acute skin infections can present as a localised skin infection, deeper cellulitis, or abscess formation31 Symptoms typically develop over three to seven days and include redness, heat, pain, inflammation and swelling32 A nodule or pustule may form and systemic symptoms of infection may also be present.
Early infection post-dermal filler is typically due to a low-grade bacterial infection from skin micro-organisms such as Staphylococcus aureus, Staphylococcus epidermidis and Propionibacterium spp. that invade the injection site32 Sebaceous glands are known to have a significant biofilm burden; this is another potential source of contamination for filler material.⁴ An early onset inflammatory nodule that is red and painful should be treated as infection – if left untreated an acute skin infection can lead to sepsis.
There is a substantial body of evidence to support that most complications of an infectious nature begin at the time of injecting due to inadequate infection cont30-32 There is no doubt that improved infection control strategies are required to reduce the microbial burden at the time of injecting.
“Infection control within aesthetic medicine must be taken very seriously; the consequences of inaction could prove to be catastrophic or indeed, fatal”
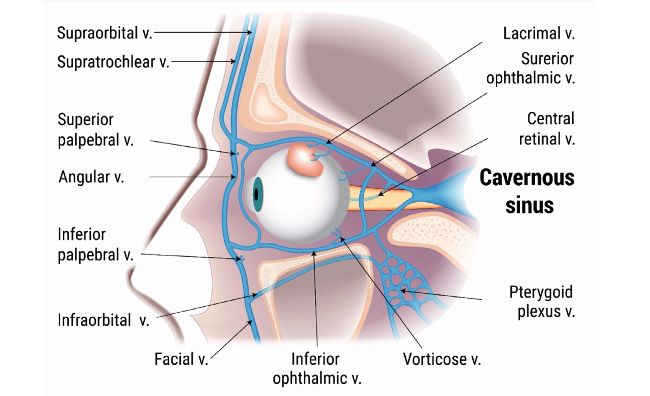
Figure 2: Location of the cavernous sinus
Delayed onset nodules
A delayed onset nodule may be a visible or palpable mass at or close to the injection site of dermal filler48 They may present as nodules, indurations, type 4 delayed hypersensitivity reactions, biofilms, abscesses, or granulomas. Symptoms can present weeks or months postinjection.
Most late and delayed onset inflammatory and nodular complications post dermal filler have an infectious origin50 It is widely accepted that biofilm infection is responsible for most delayed complications post dermal filler and the leading cause of device associated infections. Biofilms can exist for a long period of time in a dormant state, but reactivation of infection can be triggered by trauma such as more dermal filler being injected close to the biofilm, dental treatments, haematogenic infection or iatrogenic manipulation.
Minimising patient risk of infection
Infection control is key to minimising the risk of infection to the patient, however, there are other factors to consider prior to injecting. The practitioner must elicit a full medical, cosmetic, drug and allergy history, incorporating information about any previous dermal filler treatments or complications and where possible establishing the type or brand of product/s injected27 Periodontal health should be part of the medical history.
The injection of dermal filler over a permanent implant poses the risk of contamination to the existing implant with the potential to cause an infection such as abscess formation52 It is pertinent to note that the number of microbes required to cause a clinical infection is dramatically reduced from 1,000,000 to 100 per gram of tissue in the presence of a foreign body, such as dermal filler.
A patient with an active skin infection is not a suitable candidate for dermal filler treatment. Similarly, inflammatory skin conditions require careful consideration and caution30
The literature supports that inadequate infection control within the clinic setting is contributing to the development of dermal-filler associated infections27 However, the recommended guidance below is relevant to all medical aesthetic procedures that breach the skin. It is hoped that in the future as regulation within our sector improves, a formalised infection control programme specific to aesthetic medicine is developed and a standardised method of reporting infection is created.
“The literature supports that inadequate infection control within the clinic setting is contributing to the development of dermal-filler associated infections”
Evidence-based guidance
• Environmental disinfection with a 70% alcohol based hard surface disinfectant.
• Patient’s skin cleansed and all traces of make-up removed prior to any procedure that breaches the skin.
• Patient’s hair covered (if appropriate to procedure).
• Skin disinfected with a hypochlorous acidbased product (before, during and after procedure).
• Practitioner’s hands washed with Hibiscrub before and after every patient contact and before the application of gloves and on removal of gloves.
• Sterile nitrile gloves donned for all procedures that breach the skin.
• Aseptic technique executed for all procedures that breach the skin.
• Wrist and hand jewellery removed, fingernails clean, short and free from nail polish, hair tied back (if appropriate) and disposable single use apron adorned during aseptic technique.
• Bare below the elbow rule must be adhered to when executing an aseptic technique.
Covid-19: Additional infection control measures
In addition to the above, practitioners must also don a 3-ply surgical mask for low-risk procedures and an N95/FFP2 respirator mask for medium and high-risk procedures as per consensus guidance.⁵ Eye protection (face shield/goggles) must also be worn for all categories of procedures as per consensus guidance.⁵
In response to the current global pandemic of the SARS-CoV-2 virus that causes covid-19 infection, additional infection control measures must be implemented within the medical aesthetic clinic for the entire duration of the pandemic. COVID-19 Pandemic: Consensus Guidelines for Preferred Practices in an Aesthetic Clinic (Kapoor et al 2020)⁵ have been produced by ten global experts to provide practitioner guidance on enhanced infection control measures during the pandemic. There is compelling evidence to support the use of face masks and eye protection in the healthcare setting53
The expert consensus group have categorised medical aesthetic procedures into low risk, moderate risk and high risk based on the likelihood of transmission of SARS-CoV-2 virus from the patient to the practitioner. Other factors taken in account by the consensus group were aerosol generating procedures (high risk) versus non aerosol generating (lower risk), which part of the body is being treated and duration of treatment, with appropriate evidencebased guidance given. Furthermore, air purifiers with a HEPA filter are discussed and recommended as an important enhanced infection control measure. The author advises that practitioners access these guidelines to further enhance infection control measures in the clinic environment.

REFERENCES
1. World Health Organisation: Infection prevention and control (online) https://www. who.int/infection-prevention/en/
2. Rutterford L. Infection Control. Aesthetics Journal 2017; 5th May
3. Newman T. Bacteria are becoming resistant to alcohol-based disinfectants (2017) (online) www.medicalnewstoday.com
4. Nield D. Superbugs are growing more resistant to alcohol (2018) (online) www. sciencealert.com
5. Kapoor et al. COVID-19 Pandemic: Consensus Guidelines for Preferred Practices in an Aesthetic Clinic. Dermatologic Therapy 2020; 33(4): 4 July/August 2020
6. NIPCM 2012 National Infection Prevention and Control Manual (2012) (online) www. nipcm.hps.scot.nhs.uk
7. The code of practice in the prevention and control of infection and related guidance (2015) https://www.gov.uk/government/consultations/prevention-and-control-ofinfections-code-of-practice
8. Rutala WA, Weber DJ. Disinfection, sterilisation and anti-sepsis: an overview Am J infect Control 2016;44: e1 – e6
9. Edmonds SL, Zapka C, Kasper D, Gerber R, McCormack R, Macing D, et al. Effectiveness of hand hygiene for removal of Clostridium difficile spores from hands. Infect Control Hosp Epidemiol. 2013;34: 302–305
10. World Health Organisation: Infection Prevention and Control of Epidemicand Pandemic Prone Acute Respiratory Infections in Health Care. (online) http://158.232.12.119/csr/bioriskreduction/infection_control/publication/en/
11. Phillips K, Scott D, Wang YI. US Food and Drug Administration: Dermal Filler Materials, Injections, Methods and Skin Preparation. Plastic and Reconstructive Surgery: 2017; 140(4): 632 -633
12. Ayliffe GA. Role of the environment of the operating suite in surgical wound infections. Clinical Infectious Diseases 1991;13(10): 800 – 804
13. Eggers M. Infectious Disease Management and Control with Povidone Iodine. Infect Dis Ther 2019;8(4): 595
14. Haley CE, Marling-Carson M, Smith JW, Luby JP, Mackwiak PA. Bactericidal Activity of Antiseptics Against Methicillin-Resistant Staphylococcus Aureus. Journal of Clinical Microbiology 1985: 991 – 992
15. Fleischer W, Reimer K. Povidone Iodine in antisepsis – state of the art. Dermatology 1997;195 (suppl 2): 3 – 9
16. Harke P. Disinfectant: Ullmann’s Encyclopaedia of industrial chemistry Wiley VCH, 2000
17. Horner et al. Reduced susceptibility to chlorhexidine in staphylococci: is it increasing and does it matter? Journal of Antimicrobial Chemotherapy 2012;67(11): 2547 – 2559
18. Milstone AM, Pasinetti CL, Perl TM. Chlorhexidine: expanding the armamentarium for infection control and prevention. Clin Infect Dis 2008;46(2): 274- 281
19. McDonnel G, Russell D. Antiseptics and disinfectants: activity, action and resistance. Clin Microbiol Rev 1999;12(1): 147 – 79
20. ASCIA (Australian society of clinical immunology and allergy) (2017) Chlorhexidine Allergy (online) www.allergy.org.au
21. Wand ME, Bock LJ, Bonney LC, Sutton JM. Mechanisms of increased resistance to chlorhexidine and cross resistance to colistin following exposure of Klebsiella pneumoniae clinical isolates to chlorhexidine. Antimicrob Agents Chemother 2016 Dec 27;61(1): e01162 – 16
22. Hashemi MM, Holden BS, Coburn J, Taylor MF, Weber S, Hilton B, Zaugg AL, McEwan C, Carson R, Andersen JL, Price JC, Deng S,Savage PB. Proteomic Analysis of Resistance of Gram-Negative Bacteria to Chlorhexidine and Impacts on Susceptibility to Colistin, Antimicrobial Peptides Savage Front Microbiol 2019;10: 210. Published online 2019 Feb 18. doi: 10.3389/fmicb.2019.00210
23. Kampf G, Kramer A. Epidemiogic Background of Hand Hygiene and Evaluation of the Most Important Agents for Scrubs and Rubs. Clin Microbiol Reviews October 2004: 863 – 893
24. Walker R (2018) CEO Clinical health technologies
25. Bowes L (2016) Could a technology from the past change skin disinfection for the future? PMFA news;4(6) (online) www.pmfanews.com
26. Wang L, Bassiri M, Najafi R et al. Hypochlorous acid as a potential wound care agent: part 1 stabilised hypochlorous acid: a component of the inorganic armamentarium of innate immunity. J of Burns Wounds 2007 April 11;6: e5
27. Collier H. Infection Control in Aesthetic Medicine and the consequences of inaction. J of Aesthetic Nursing 2018;Vol 7(7): 352
28. Bik EM et al. Bacterial diversity in the oral cavity of ten healthy individuals. ISME J 2010 Aug;4(8): 962-74
29. Hermesch CB, Hilton TJ, Biesbrock AR et al. Perioperative use of chlorhexidine gluconate for the prevention of alveolar osteitis: efficacy and risk factor analysis. Oral Surg Oral Med Oral pathol Oral Radiol Endod 1998;85: 381-387
30. De Boulle K. Patient factors influencing dermal filler complications: prevention, assessment and treatment. Clin Cosmet Investig Dermatol 2015;(8): 205 – 214
31. Wagner et al. Aetiology, Prevention, and Management of Infectious Complications of Dermal Fillers. Semin Plast Surg 2016;30: 83 – 86
32. Ferneini et al. An Overview of Infections Associated with Soft Tissue Facial Fillers: Identification, Prevention and treatment. J Oral Maxillofacial Surg 2017;75: 160 – 166
33. Signorini et al. Global Aesthetics Consensus: Avoidance and Management of Complications from Hyaluronic Acid Fillers – Evidence and Opinion Based Review and Consensus Recommendations. Plastic and Reconstructive Surgery June 2016;6: 961e – 965e
34. Matewele P. Out of date make up can contain lethal bacteria. London Metropolitan University (2015) (online) www.londonmet.ac.uk/news/articles/mke-up
35. Mathur P. Hand Hygiene: Back to the basics of infection control. The Indian Journal of Medical Research 2011;134(5): 611 – 620
36. Nice Guideline (2012) Healthcare associated infection: prevention and control in primary and community care. (online) www.nice.org.uk
37. NHS, National Services Scotland, Infection Control Team (2016) Standard Infection Control Precautions (SICPs) Literature Review: Personal Protective Equipment – Gloves Health Protection Scotland Version 3.0 July 2016
38. Pittet D. Improving adherence to hand hygiene practices: a multi-disciplinary approach. Emerging Infectious Diseases 2001;7(2): 234 – 24
39. Fuller C, Savage J, Besser S, Hayward A, Cookson B, Cooper B, Stone S. “The Dirty Hand in the Latex Glove”: A Study of Hand Hygiene Compliance When Gloves are Worn. Infection Control and Hospital Epidemiology 2011 Dec;32(12): 1194 – 9 40. Flores A, Pevalin D. Glove use and compliance with hand hygiene. Nursing Times 2007; 103(38): 46 – 48
41. Mansouri M, Tidley M, Sanati KA, Roberts CA. Comparison of blood transmission through latex and nitrile glove materials. Occup Med (Lond) 2010 May;60(3): 205 – 2010
42. Ferneini et al. An Overview of Infections Associated with Soft Tissue Facial Fillers: Identification, Prevention and treatment. J Oral Maxillofacial Surg 2017;75: 160 – 166
43. Narins S. Biofilms from dermal fillers management with proper course of action. (2011) http://tinyurl.com [Accessed May 2018]
44. Chen et al. Septic Cavernous Sinus Thrombosis: An unusual and fatal disease. J Formos Med Assoc Mar 2006:105(3); 203 – 209
45. Chick et al. Bilateral cavernous sinus thrombosis following community acquired methicillin resistant staphylococcus aureus infection: A case report and review of the literature. J Miss State Med Assoc 2010 Nov;51(11): 317-20
46. Ashkenazi et al. Eradication of Propionibacterium acnes by its endogenic porphyrins after illumination with high intensity blue light. FEMS Immunol. Med Microbiol 2003;35: 17 – 24
47. Sclafani AP, Fagan S. Treatment of injectable soft tissue filler complications. Dermatol Surg 2009;35(suppl 2): 1672 – 80
48. Signorini et al. Global Aesthetics Consensus: Avoidance and Management of Complications from Hyaluronic Acid Fillers – Evidence and Opinion Based Review and Consensus Recommendations. Plastic and Reconstructive Surgery June 2016;6: 961e – 965e
49. Saththianathan et al. The Role of Bacterial Biofilm in Adverse Soft Tissue Filler Reactions: A combined Laboratory and Clinical Study. Plast and Reconstr Surg 2017;139(3): 613 – 621
50. Dumitrascu D, Georgescu A. The management of biofilm formation after hyaluronic acid gel filler injections: A review Clujul Medical 2013;86(3): 192 – 195
51. Narins S (2011) Biofilms from dermal fillers management with proper course of action. (2011) http://tinyurl.com [Accessed May 2018]
52. Rzany B, De Lorenzie C. Understanding, Avoiding, and Managing Severe Filler Complications. Plast Reconstr Surg 2015;136(5s): 196s – 203s
53. Chue et al. Physical distancing, face masks and eye protection to prevent person to person transmission of SARS-CoV-2 and COVID 19: a Systematic review and metaanalysis. The Lancet June 1st, 2020
54. Van Doremalen N et al. Aerosol and Surface Stability of HCoV-19 (sars-CoV2) compared to SARS CoV-1. N Engl J Med 2020;382: 1564-7
55. Homer C, Mawer D, Wilcox M. Reduced Susceptibility to chlorhexidine in staphylococcus: is it increasing and does it matter? J Antimicrob Chemother 2012;67(11): 2547 – 59
56. NHS Salisbury Foundation Trust (2015) Glove Usage Policy www.icid.salisbury.nhs. uk
57. NES (2014) Healthcare associated Infections – ASEPTIC Technique www.nes.scot. nhs.uk