INFECTION CONTROL
Controlled environment
Aesthetic nurse Helena Collier of ACE Group World (Aesthetic Complications Experts) sets out the first part of the Group’s new guidelines on infection control in aesthetic medicine
DEFINITION
Infection prevention and control is a scientific approach and practical solution designed to prevent harm caused by infection to patients and healthcare workers.1
INFECTION CONTROL IN AESTHETIC MEDICINE
ACE Group World has produced a series of evidence-based and peer-reviewed guidelines to help practitioners prevent and manage complications that can occur in aesthetic practice. These guidelines are not intended to replace clinical judgement and it is important the practitioner makes the correct diagnosis and works within their scope of competency. Some complications may require prescription medicines to help in their management and if the practitioner is not familiar with the medication, the patient should be appropriately referred.
Informing the patient’s general practitioner is considered good medical practice and patient consent should be sought. It may be appropriate to involve the general practitioner or other specialist for shared care management when the treating practitioner is not able or lacks experience to manage the complication themselves. Practitioners have a duty of care and are accountable to their professional bodies and must act honestly, ethically, and professionally.
OVERVIEW
Microbiology is the study of microbes. Microbes are living organisms so small they can only be seen through a microscope. They are considered the smallest form of life and include bacteria, viruses, fungi, archaea, and protozoa. Microbes that can cause disease are referred to as pathogens. The relationship between the human body and the microbial world is truly dynamic. However, despite this lifelong partnership and the undeniable value these organisms can bring to the human body and the earth’s ecology, pathogens are capable of destroying human life.
The skin and mucous membranes are the body’s protective barrier – if this defence is breached by pathogens they can reach subcutaneous tissue, muscle, bone, and body cavities. In the field of aesthetic medicine, the injection of dermal filler into soft tissue is one of the most sought-after treatments. This procedure can incorporate multiple injection passes from skin to bone. There is a risk of an infectious complication arising from any medical aesthetic procedure that breaches the skin; however, the injection of dermal filler poses a higher risk to patients if strict infection control measures are not adhered to (Table 1).
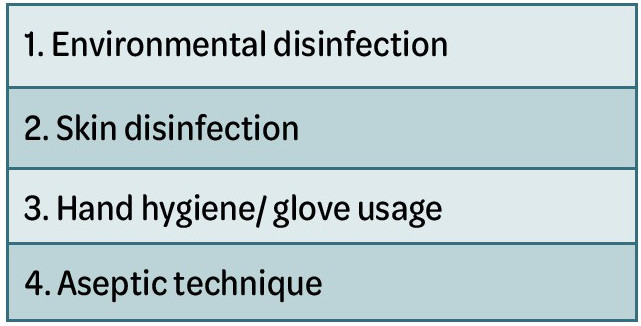
Table 1: Infection control measures
These guidelines will offer evidencebased guidance on infection control within aesthetic medicine and explore the potential complications that can result as a consequence of inaction. All healthcare professionals (HCPs) have a duty to protect patients from harm. Infection prevention and control is the responsibility of everyone involved in the delivery of aesthetic medicine.
ENVIRONMENTAL DISINFECTION
As patients and visitors enter the clinic setting, they bring with them their own unique microbiota. As inanimate objects such as door handles, stair rails, surfaces, taps and pens, etc. are inevitably touched, contamination and an exchange of microbes is occurring.2
The practitioner delivering patient care is also harbouring a frenzy of living organisms on their being and must be vigilant with hand washing and asepsis. Microbes on the skin can be killed with the use of antiseptics and surface contamination eradicated with disinfectants. However, over recent years, researchers have noted a steady rise in drug-resistant bacteria such as enterococcus faecium and vancomycinresistant enterococci spp. growing a tolerance to alcohol-based disinfectant.2-4
These findings are more relevant to hospital-acquired infections, but drugresistant bacteria are now becoming more prevalent in our communities.4 Anti-microbial resistance is one of the greatest threats to human life. In the clinic environment, if surfaces are not kept scrupulously clean there is a risk of contamination and cross-infection. In a healthcare facility the source of infection may be the staff, the patient, or the environment. Multiple pathogens can be found on inanimate objects and some have the potential to survive up to four years2 (Table 2).
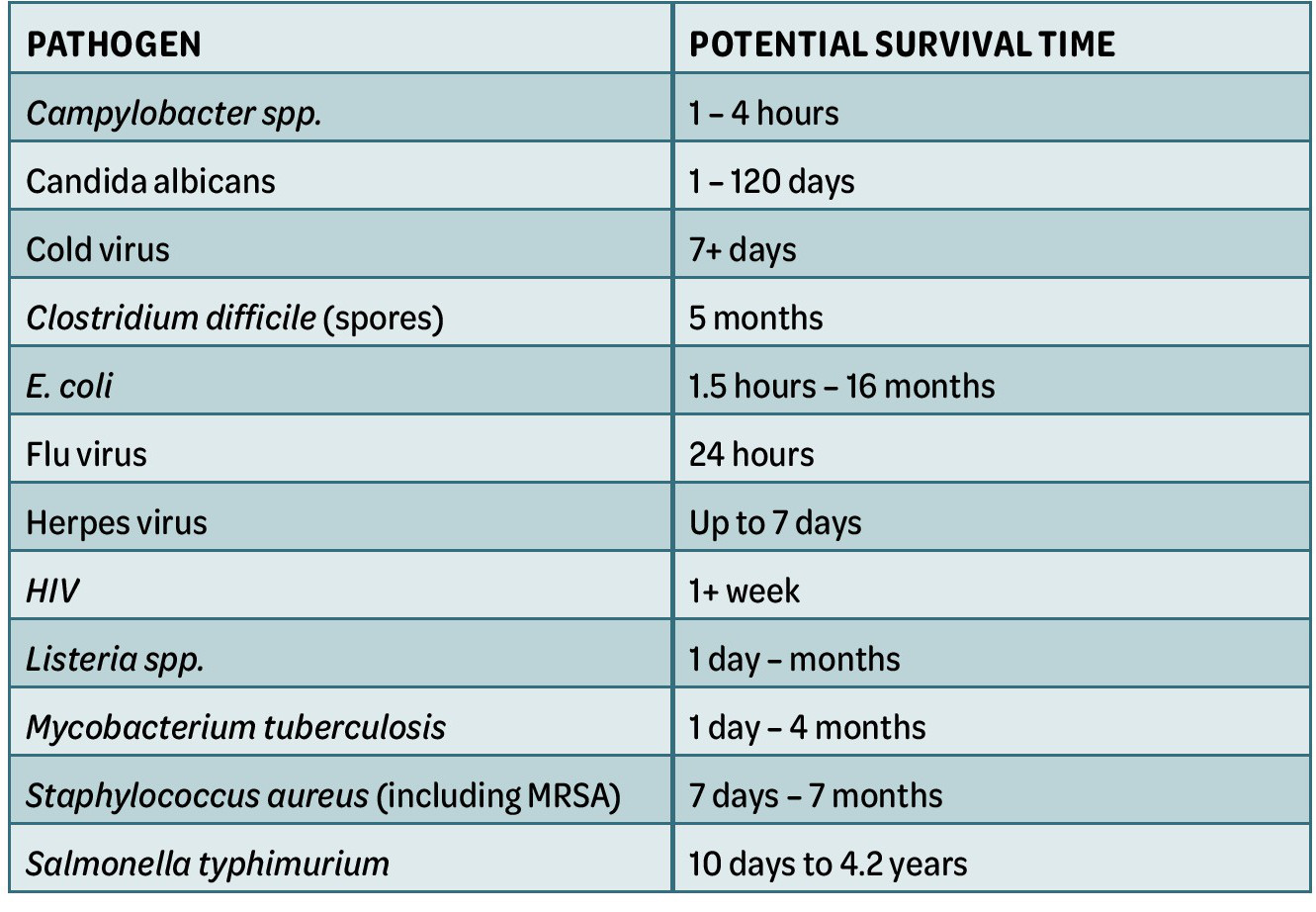
Table 2: Some of the most common types of pathogens and how long they can survive on dry surfaces
With reference to the current global pandemic, scientists have found that SARS– CoV-2 (the virus that causes covid-19) can be detected on plastic and stainless-steel surfaces for up to three days.54
Cleaning and disinfecting surfaces in a healthcare setting is fundamental to reduce the risk of infection. As well as scrupulous general cleaning of the clinic environment, disinfection of the treatment chair/ couch before and after each patient is essential practice, as is disinfecting hard surfaces and other touched surfaces in the treatment area before and after each procedure.
Practitioners can be further guided by the National Infection Prevention and Control Manual (2012)6 or the Code of practice on the prevention and control of infections and related guidance (2015).
Alcohol 70%
Most hard-surface disinfectants are based on isopropyl or ethanol alcohol 70%, in the form of impregnated wipes or spray. Hard surfaces must be clean and free of contaminants for alcohol to have any microbial effect.8
The key benefit of alcohol 70% is broad-spectrum activity against a range of pathogenic micro-organisms including E. coli, pseudomonas aeruginosa, staphylococcus aureus, enterococci spp., methicillin-resistant staphylococcus aureus, hepatitis B and C, HIV and candida albicans.8 However, alcohol-based disinfectants are less effective against norovirus and ineffective against spores.9 Nonetheless, alcohol-based solutions have been shown to be effective in disinfecting surfaces contaminated with pandemic viruses such as eboli and SARS coronavirus. Alcohol is highly flammable, therefore its use should be limited to small surface areas in well ventilated spaces.10
Bleach (active ingredient: sodium hypochlorite)
Bleach is an alternative chemical to alcohol as a surface disinfectant. It is effective in killing bacteria, fungi and viruses but is easily inactivated by organic material, such as blood. At high concentration, bleach can kill spores (such as C. Difficile), however, it irritates mucous membranes, the skin and the airways and can be highly corrosive to surfaces.10
SKIN DISINFECTION
Interrupting pathogenesis is key to reducing infections associated with injectable treatments in the field of aesthetic medicine. Although infections are infrequent, they can have a devastating impact on quality of life.11 Antiseptic skin disinfection and aseptic technique play a pivotal role in limiting the transfer of pathogens. Skin disinfection is a process that involves the topical application of a disinfectant to the entire area, not just the site of injection, to reduce levels of microorganisms prior to a procedure that breaches the skin.
Most surgical site infections are said to originate from the patient’s own bacteria entering the wound at the time of the procedure.12 This supports the need for stringent skin disinfection and the execution of aseptic technique during procedures that breach the skin – the injection of dermal filler is no exception.
Evaluation of frequently-used skin disinfectants:
“Infection prevention and control is the responsibility of everyone involved in the delivery of aesthetic medicine”
In 2007 the Food and Drug Administration (FDA) issued a warning to highlight the increase in adverse events seen with chlorhexidine exposure, some resulting in anaphylaxis.
“Interrupting pathogenesis is key to reducing infections associated with injectable treatments”
Overuse of antibiotics has led to the emergence of superbugs and a whole new world of antibiotic-resistant microbes. There is a risk that medicine could return to its pre-antibiotic era if new defences against microbes are not found.24 The development of hypochlorous acid (HCOI)-based products offers a timely response to this need.25 The introduction of HOCl as a skin disinfectant in the field of aesthetic medicine is a major advance of the 21st century.27 It has been reported that the need for skin disinfection is as necessary as ever given the now universal understanding that even a needle-stick injury can allow the ingress of biofilm and lead to infection.21
Anti-microbial mouthwash (hypochlorous acid or chlorhexidinebased mouthwash)
It has been estimated that there are between 500 and 650 different species of micro-organisms in and around the mouth.28 An antiseptic mouthwash used prior to dermal filler injections to the lip area will reduce bacterial flora for approximately eight hours and will also minimise the risk of contamination when licking the lips.29
Dental treatments should be carried out either two weeks before or two weeks after the injection of dermal filler to reduce the risk of haematological bacterial spread.30
Make-up removal prior to skin disinfection
There is a substantial volume of reports in the literature about the absolute necessity to remove make-up from the skin before the injection of dermal filler, however, the evidence is predominantly consensus of expert opinion.30-33 A study commissioned by Rakish Aggrawal, chief executive of online cosmetic company escentual.com, reports findings of concern.³⁴ Dr Paul Matewele, senior lecturer in Biomedical Science at the London Metropolitan University, who conducted the study, tested five items of makeup that were either close to the use-by date or just over, and reported that under strict laboratory conditions, all items tested positive for the bacteria enterococcus faecalis. This is a deadly strain of bacteria that can cause meningitis and septicaemia and is one of the biggest killers of new-born babies.³⁴ Other bacteria found growing in the make-up and the potential health risks are listed in Table 3.
This is evidence enough to support why the skin must be completely free of makeup, cleansed and then disinfected before the injection of dermal filler. The skin must remain free from make-up for 12 hours postinjection.
There are no universal guidelines for skin disinfection prior to the injection of dermal filler; this is a major gap in global patient health and safety.27
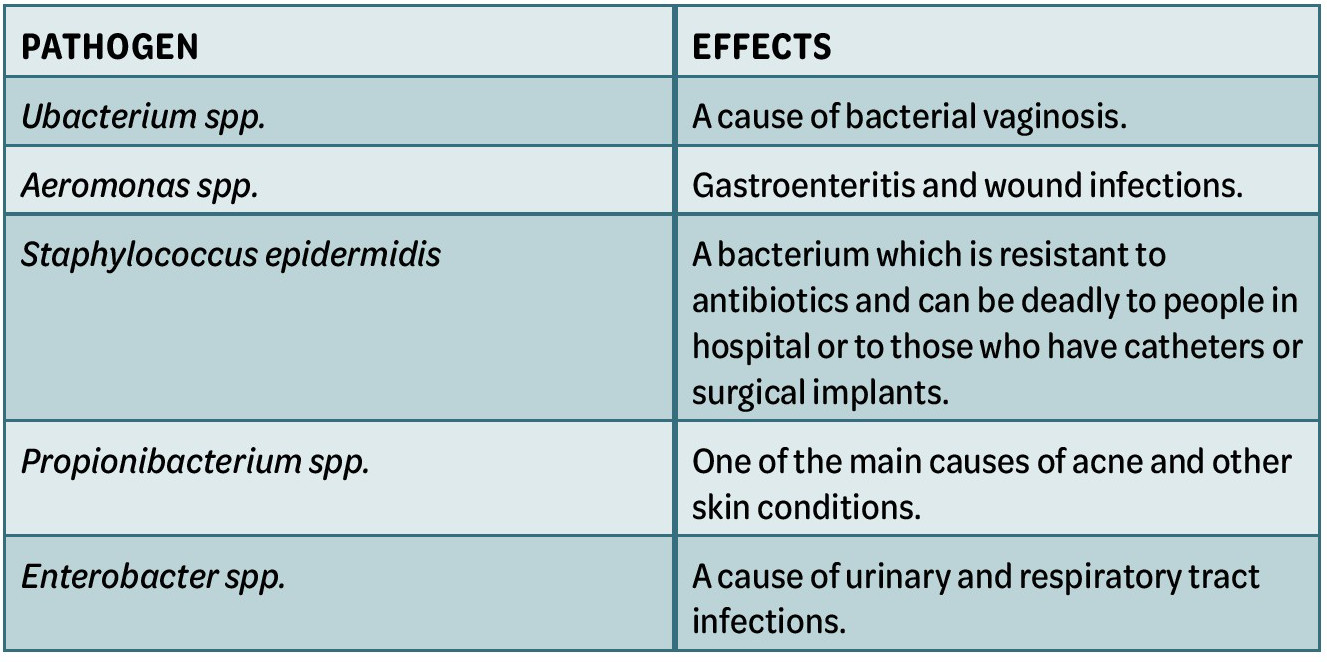
Table 3: Contaminated make-up study34
REFERENCES
1. World Health Organisation: Infection prevention and control (online) https://www. who.int/infection-prevention/en/
2. Rutterford L. Infection Control. Aesthetics Journal 2017; 5th May
3. Newman T. Bacteria are becoming resistant to alcohol-based disinfectants (2017) (online) www.medicalnewstoday.com
4. Nield D. Superbugs are growing more resistant to alcohol (2018) (online) www. sciencealert.com
5. Kapoor et al. COVID-19 Pandemic: Consensus Guidelines for Preferred Practices in an Aesthetic Clinic. Dermatologic Therapy 2020; 33(4): 4 July/August 2020
6. NIPCM 2012 National Infection Prevention and Control Manual (2012) (online) www. nipcm.hps.scot.nhs.uk
7. The code of practice in the prevention and control of infection and related guidance (2015) https://www.gov.uk/government/consultations/prevention-and-control-ofinfections-code-of-practice
8. Rutala WA, Weber DJ. Disinfection, sterilisation and anti-sepsis: an overview Am J infect Control 2016;44: e1 – e6
9. Edmonds SL, Zapka C, Kasper D, Gerber R, McCormack R, Macing D, et al. Effectiveness of hand hygiene for removal of Clostridium difficile spores from hands. Infect Control Hosp Epidemiol. 2013;34: 302–305
10. World Health Organisation: Infection Prevention and Control of Epidemicand Pandemic Prone Acute Respiratory Infections in Health Care. (online) http://158.232.12.119/csr/bioriskreduction/infection_control/publication/en/
11. Phillips K, Scott D, Wang YI. US Food and Drug Administration: Dermal Filler Materials, Injections, Methods and Skin Preparation. Plastic and Reconstructive Surgery: 2017; 140(4): 632 -633
12. Ayliffe GA. Role of the environment of the operating suite in surgical wound infections. Clinical Infectious Diseases 1991;13(10): 800 – 804
13. Eggers M. Infectious Disease Management and Control with Povidone Iodine. Infect Dis Ther 2019;8(4): 595
14. Haley CE, Marling-Carson M, Smith JW, Luby JP, Mackwiak PA. Bactericidal Activity of Antiseptics Against Methicillin-Resistant Staphylococcus Aureus. Journal of Clinical Microbiology 1985: 991 – 992
15. Fleischer W, Reimer K. Povidone Iodine in antisepsis – state of the art. Dermatology 1997;195 (suppl 2): 3 – 9
16. Harke P. Disinfectant: Ullmann’s Encyclopaedia of industrial chemistry Wiley VCH, 2000
17. Horner et al. Reduced susceptibility to chlorhexidine in staphylococci: is it increasing and does it matter? Journal of Antimicrobial Chemotherapy 2012;67(11): 2547 – 2559
18. Milstone AM, Pasinetti CL, Perl TM. Chlorhexidine: expanding the armamentarium for infection control and prevention. Clin Infect Dis 2008;46(2): 274-281
19. McDonnel G, Russell D. Antiseptics and disinfectants: activity, action and resistance. Clin Microbiol Rev 1999;12(1): 147 – 79
20. ASCIA (Australian society of clinical immunology and allergy) (2017) Chlorhexidine Allergy (online) www.allergy.org.au
21. Wand ME, Bock LJ, Bonney LC, Sutton JM. Mechanisms of increased resistance to chlorhexidine and cross resistance to colistin following exposure of Klebsiella pneumoniae clinical isolates to chlorhexidine. Antimicrob Agents Chemother 2016 Dec 27;61(1): e01162 – 16
22. Hashemi MM, Holden BS, Coburn J, Taylor MF, Weber S, Hilton B, Zaugg AL, McEwan C, Carson R, Andersen JL, Price JC, Deng S,Savage PB. Proteomic Analysis of Resistance of Gram-Negative Bacteria to Chlorhexidine and Impacts on Susceptibility to Colistin, Antimicrobial Peptides Savage Front Microbiol 2019;10: 210. Published online 2019 Feb 18. doi: 10.3389/fmicb.2019.00210
23. Kampf G, Kramer A. Epidemiogic Background of Hand Hygiene and Evaluation of the Most Important Agents for Scrubs and Rubs. Clin Microbiol Reviews October 2004: 863 – 893
24. Walker R (2018) CEO Clinical health technologies
25. Bowes L (2016) Could a technology from the past change skin disinfection for the future? PMFA news;4(6) (online) www.pmfanews.com
26. Wang L, Bassiri M, Najafi R et al. Hypochlorous acid as a potential wound care agent: part 1 stabilised hypochlorous acid: a component of the inorganic armamentarium of innate immunity. J of Burns Wounds 2007 April 11;6: e5
27. Collier H. Infection Control in Aesthetic Medicine and the consequences of inaction. J of Aesthetic Nursing 2018;Vol 7(7): 352
28. Bik EM et al. Bacterial diversity in the oral cavity of ten healthy individuals. ISME J 2010 Aug;4(8): 962-74
29. Hermesch CB, Hilton TJ, Biesbrock AR et al. Perioperative use of chlorhexidine gluconate for the prevention of alveolar osteitis: efficacy and risk factor analysis. Oral Surg Oral Med Oral pathol Oral Radiol Endod 1998;85: 381-387
30. De Boulle K. Patient factors influencing dermal filler complications: prevention, assessment and treatment. Clin Cosmet Investig Dermatol 2015;(8): 205 – 214
31. Wagner et al. Aetiology, Prevention, and Management of Infectious Complications of Dermal Fillers. Semin Plast Surg 2016;30: 83 – 86
32. Ferneini et al. An Overview of Infections Associated with Soft Tissue Facial Fillers: Identification, Prevention and treatment. J Oral Maxillofacial Surg 2017;75: 160 – 166
33. Signorini et al. Global Aesthetics Consensus: Avoidance and Management of Complications from Hyaluronic Acid Fillers – Evidence and Opinion Based Review and Consensus Recommendations. Plastic and Reconstructive Surgery June 2016;6: 961e – 965e
34. Matewele P. Out of date make up can contain lethal bacteria. London Metropolitan University (2015) (online) www.londonmet.ac.uk/news/articles/mke-up
35. Mathur P. Hand Hygiene: Back to the basics of infection control. The Indian Journal of Medical Research 2011;134(5): 611 – 620
36. Nice Guideline (2012) Healthcare associated infection: prevention and control in primary and community care. (online) www.nice.org.uk
37. NHS, National Services Scotland, Infection Control Team (2016) Standard Infection Control Precautions (SICPs) Literature Review: Personal Protective Equipment – Gloves Health Protection Scotland Version 3.0 July 2016
38. Pittet D. Improving adherence to hand hygiene practices: a multi-disciplinary approach. Emerging Infectious Diseases 2001;7(2): 234 – 24
39. Fuller C, Savage J, Besser S, Hayward A, Cookson B, Cooper B, Stone S. “The Dirty Hand in the Latex Glove”: A Study of Hand Hygiene Compliance When Gloves are Worn. Infection Control and Hospital Epidemiology 2011 Dec;32(12): 1194 – 9
40. Flores A, Pevalin D. Glove use and compliance with hand hygiene. Nursing Times 2007; 103(38): 46 – 48
41. Mansouri M, Tidley M, Sanati KA, Roberts CA. Comparison of blood transmission through latex and nitrile glove materials. Occup Med (Lond) 2010 May;60(3): 205 – 2010
42. Ferneini et al. An Overview of Infections Associated with Soft Tissue Facial Fillers: Identification, Prevention and treatment. J Oral Maxillofacial Surg 2017;75: 160 – 166
43. Narins S. Biofilms from dermal fillers management with proper course of action. (2011) http://tinyurl.com [Accessed May 2018]
44. Chen et al. Septic Cavernous Sinus Thrombosis: An unusual and fatal disease. J Formos Med Assoc Mar 2006:105(3); 203 – 209
45. Chick et al. Bilateral cavernous sinus thrombosis following community acquired methicillin resistant staphylococcus aureus infection: A case report and review of the literature. J Miss State Med Assoc 2010 Nov;51(11): 317-20
46. Ashkenazi et al. Eradication of Propionibacterium acnes by its endogenic porphyrins after illumination with high intensity blue light. FEMS Immunol. Med Microbiol 2003;35: 17 – 24
47. Sclafani AP, Fagan S. Treatment of injectable soft tissue filler complications. Dermatol Surg 2009;35(suppl 2): 1672 – 80
48. Signorini et al. Global Aesthetics Consensus: Avoidance and Management of Complications from Hyaluronic Acid Fillers – Evidence and Opinion Based Review and Consensus Recommendations. Plastic and Reconstructive Surgery June 2016;6: 961e – 965e
49. Saththianathan et al. The Role of Bacterial Biofilm in Adverse Soft Tissue Filler Reactions: A combined Laboratory and Clinical Study. Plast and Reconstr Surg 2017;139(3): 613 – 621
50. Dumitrascu D, Georgescu A. The management of biofilm formation after hyaluronic acid gel filler injections: A review Clujul Medical 2013;86(3): 192 – 195
51. Narins S (2011) Biofilms from dermal fillers management with proper course of action. (2011) http://tinyurl.com [Accessed May 2018]
52. Rzany B, De Lorenzie C. Understanding, Avoiding, and Managing Severe Filler Complications. Plast Reconstr Surg 2015;136(5s): 196s – 203s
53. Chue et al. Physical distancing, face masks and eye protection to prevent person to person transmission of SARS-CoV-2 and COVID 19: a Systematic review and metaanalysis. The Lancet June 1st, 2020
54. Van Doremalen N et al. Aerosol and Surface Stability of HCoV-19 (sars-CoV2) compared to SARS CoV-1. N Engl J Med 2020;382: 1564-7
55. Homer C, Mawer D, Wilcox M. Reduced Susceptibility to chlorhexidine in staphylococcus: is it increasing and does it matter? J Antimicrob Chemother 2012;67(11): 2547 – 59
56. NHS Salisbury Foundation Trust (2015) Glove Usage Policy www.icid.salisbury.nhs.uk
57. NES (2014) Healthcare associated Infections – ASEPTIC Technique www.nes.scot.nhs.uk